Highly efficient Agrobacterium rhizogenes-mediated hairy root transformation in citrus seeds and its application in gene functional analysis
- PMID: 38023879
- PMCID: PMC10644275
- DOI: 10.3389/fpls.2023.1293374
Highly efficient Agrobacterium rhizogenes-mediated hairy root transformation in citrus seeds and its application in gene functional analysis
Abstract
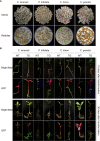
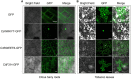
Highly efficient genetic transformation technology is beneficial for plant gene functional research and molecular improvement breeding. However, the most commonly used Agrobacterium tumefaciens-mediated genetic transformation technology is time-consuming and recalcitrant for some woody plants such as citrus, hampering the high-throughput functional analysis of citrus genes. Thus, we dedicated to develop a rapid, simple, and highly efficient hairy root transformation system induced by Agrobacterium rhizogenes to analyze citrus gene function. In this report, a rapid, universal, and highly efficient hairy root transformation system in citrus seeds was described. Only 15 days were required for the entire workflow and the system was applicable for various citrus genotypes, with a maximum transformation frequency of 96.1%. After optimization, the transformation frequency of Citrus sinensis, which shows the lowest transformation frequency of 52.3% among four citrus genotypes initially, was increased to 71.4% successfully. To test the applicability of the hairy roots transformation system for gene functional analysis of citrus genes, we evaluated the subcellular localization, gene overexpression and gene editing in transformed hairy roots. Compared with the traditional transient transformation system performed in tobacco leaves, the transgenic citrus hairy roots displayed a more clear and specific subcellular fluorescence localization. Transcript levels of genes were significantly increased in overexpressing transgenic citrus hairy roots as compared with wild-type (WT). Additionally, hairy root transformation system in citrus seeds was successful in obtaining transformants with knocked out targets, indicating that the Agrobacterium rhizogenes-mediated transformation enables the CRISPR/Cas9-mediated gene editing. In summary, we established a highly efficient genetic transformation technology with non-tissue-culture in citrus that can be used for functional analysis such as protein subcellular localization, gene overexpression and gene editing. Since the material used for genetic transformation are roots protruding out of citrus seeds, the process of planting seedlings prior to transformation of conventional tissue culture or non-tissue-culture was eliminated, and the experimental time was greatly reduced. We anticipate that this genetic transformation technology will be a valuable tool for routine research of citrus genes in the future.
Keywords: Agrobacterium rhizogenes; citrus; gene editing; genetic transformation; subcellular localization.
Copyright © 2023 Wang, Qin, Wei, Xue and Dai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Basu S., Sineva E., Nguyen L., Sikdar N., Park J. W., Sinev M., et al. . (2022). Host-derived chimeric peptides clear the causative bacteria and augment host innate immunity during infection: A case study of HLB in citrus and fire blight in apple. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.929478 - DOI - PMC - PubMed
-
- Castellanos-Arévalo A. P., EstradaLuna A. A., CabreraPonce J. L., ValenciaLozano E., de HerreraUbaldo H., Folter. S., et al. . (2020). Agrobacterium rhizogenes-mediated transformation of grain (Amaranthus hypochondriacus) and leafy (A. hybridus) amaranths. Plant Cell Rep. 39 (9), 1143–1160. doi: 10.1007/s00299-020-02553-9 - DOI - PubMed
-
- Dahro B., Li C. L., Liu J. H. (2023). Overlapping responses to multiple abiotic stresses in citrus: from mechanism understanding to genetic improvement. Hortic. Adv. 1, 4. doi: 10.1007/s44281-023-00007-2 - DOI
-
- Dai W. S., Peng T., Wang M., Liu J. H. (2023). Genome-wide identification and comparative expression profiling of the WRKY transcription factor family in two Citrus species with different Candidatus Liberibacter Asiaticus susceptibility. BMC Plant Biol. 23 (1), 159. doi: 10.1186/s12870-023-04156-4 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

