Magnetic Hydrogel Beads as a Reusable Adsorbent for Highly Efficient and Rapid Removal of Aluminum: Characterization, Response Surface Methodology Optimization, and Evaluation of Isotherms, Kinetics, and Thermodynamic Studies
- PMID: 38024693
- PMCID: PMC10652826
- DOI: 10.1021/acsomega.3c04984
Magnetic Hydrogel Beads as a Reusable Adsorbent for Highly Efficient and Rapid Removal of Aluminum: Characterization, Response Surface Methodology Optimization, and Evaluation of Isotherms, Kinetics, and Thermodynamic Studies
Abstract
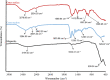
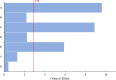
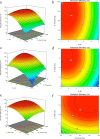
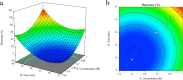
Biopolymers such as alginate and gelatin have attracted much attention because of their exceptional adsorption properties and biocompatibility. The magnetic hydrogel beads produced and used in this study had a core structure composed of magnetite nanoparticles and gelatin and a shell structure composed of alginate. The combination of the metal-ion binding ability of alginate and the mechanical strength of gelatin in magnetic hydrogel beads presents a new approach for the removal of metal from water sources. The beads were designed for aluminum removal and fully characterized using various methods, including Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy, scanning electron microscopy-energy-dispersive X-ray spectroscopy, vibrating sample magnetometry, microcomputed tomography, and dynamic mechanical analysis. Statistical experimental designs were employed to optimize the parameters of the adsorption and recovery processes. Plackett-Burman Design, Box-Behnken Design, and Central Composite Design were used for identifying the significant factors and optimizing the parameters of the adsorption and recovery processes, respectively. The optimum parameters determined for adsorption are as follows: pH: 4, contact time: 30 min, adsorbent amount: 600 mg; recovery time: reagent 1 M HNO3; and contact time: 40 min. The adsorption process was described by using the Langmuir isotherm model. It reveals a homogeneous bead surface and monolayer adsorption with an adsorption capacity of 5.25 mg g-1. Limit of detection and limit of quantification values were calculated as 4.3 and 14 μg L-1, respectively. The adsorption process was described by a pseudo-second-order kinetic model, which assumes that chemisorption is the rate-controlling mechanism. Thermodynamic studies indicate that adsorption is spontaneous and endothermic. The adsorbent was reusable for 10 successive adsorption-desorption cycles with a quantitative adsorption of 98.2% ± 0.3% and a recovery of 99.4% ± 2.6%. The minimum adsorbent dose was determined as 30 g L-1 to achieve quantitative adsorption of aluminum. The effects of the inorganic ions were also investigated. The proposed method was applied to tap water and carboy water samples, and the results indicate that magnetic hydrogel beads can be an effective and reusable bioadsorbent for the detection and removal of aluminum in water samples. The recovery values obtained by using the developed method were quantitative and consistent with the results obtained from the inductively coupled plasma optical emission spectrometer.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Sharma R.; Sharma K.; Kumar Saraswat B. A review of the mechanical and chemical properties of aluminium alloys AA6262 T6 and its composites for turning process in the CNC. Mater. Today: Proc. 2023, 10.1016/j.matpr.2023.01.421. - DOI
-
- Ashkenazi D. How aluminum changed the world: A metallurgical revolution through technological and cultural perspectives. Technol. Forecast. Soc. Change 2019, 143, 101–113. 10.1016/j.techfore.2019.03.011. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

