Bacterial Nanocellulose/Copper as a Robust Laccase-Mimicking Bionanozyme for Catalytic Oxidation of Phenolic Pollutants
- PMID: 38024715
- PMCID: PMC10652835
- DOI: 10.1021/acsomega.3c06847
Bacterial Nanocellulose/Copper as a Robust Laccase-Mimicking Bionanozyme for Catalytic Oxidation of Phenolic Pollutants
Abstract
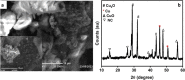
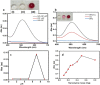
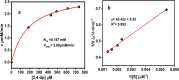
Industrial effluents containing phenolic compounds are a major public health concern and thus require effective and robust remediation technologies. Although laccase-like nanozymes are generally recognized as being catalytically efficient in oxidizing phenols, their support materials often lack resilience in harsh environments. Herein, bacterial nanocellulose (BNC) was introduced as a sustainable, strong, biocompatible, and environmentally friendly biopolymer for the synthesis of a laccase-like nanozyme (BNC/Cu). A native bacterial strain that produces nanocellulose was isolated from black tea broth fermented for 1 month. The isolate that produced BNC was identified as Bacillus sp. strain T15, and it can metabolize hexoses, sucrose, and less expensive substrates, such as molasses. Further, BNC/Cu nanozyme was synthesized using the in situ reduction of copper on the BNC. Characterization of the nanozyme by scanning electron microscopy (SEM) and X-ray diffraction (XRD) confirmed the presence of the copper nanoparticles dispersed in the layered sheets of BNC. The laccase-mimetic activity was assessed using the chromogenic redox reaction between 2,4-dichlorophenol (2,4-DP) and 4-aminoantipyrine (4-AP) with characteristic absorption at 510 nm. Remarkably, BNC/Cu has 50.69% higher catalytic activity than the pristine Cu NPs, indicating that BNC served as an effective biomatrix to disperse Cu NPs. Also, the bionanozyme showed the highest specificity toward 2,4-DP with a Km of 0.187 mM, which was lower than that of natural laccase. The bionanozyme retained catalytic activity across a wider temperature range with optimum activity at 85 °C, maintaining 38% laccase activity after 11 days and 46.77% activity after the fourth cycle. The BNC/Cu bionanozyme could efficiently oxidize more than 70% of 1,4-dichlorophenol and phenol in 5 h. Thereby, the BNC/Cu bionanozyme is described here as having an efficient ability to mimic laccase in the oxidation of phenolic compounds that are commonly released into the environment by industry.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Rostami A.; Abdelrasoul A.; Shokri Z.; Shirvandi Z. Applications and Mechanisms of Free and Immobilized Laccase in Detoxification of Phenolic Compounds — A Review. Korean J. Chem. Eng. 2022, 39 (4), 821–832. 10.1007/s11814-021-0984-0. - DOI
-
- Arregui L.; Ayala M.; Gómez-Gil X.; Gutiérrez-Soto G.; Hernández-Luna C. E.; Herrera de los Santos M.; Levin L.; Rojo-Domínguez A.; Romero-Martínez D.; Saparrat M. C. N.; Trujillo-Roldán M. A.; Valdez-Cruz N. A. Laccases: Structure, Function, and Potential Application in Water Bioremediation. Microb. Cell Fact. 2019, 18 (1), 20010.1186/s12934-019-1248-0. - DOI - PMC - PubMed
-
- Ma H.; Zheng N.; Chen Y.; Jiang L. Laccase-like Catalytic Activity of Cu-Tannic Acid Nanohybrids and Their Application for Epinephrine Detection. Colloids Surf., A 2021, 613, 12610510.1016/j.colsurfa.2020.126105. - DOI
LinkOut - more resources
Full Text Sources

