Effects of the aldehyde-derived ring substituent on the properties of two new bioinspired trimethoxybenzoylhydrazones: methyl vs nitro groups
- PMID: 38025090
- PMCID: PMC10644011
- DOI: 10.3762/bjoc.19.125
Effects of the aldehyde-derived ring substituent on the properties of two new bioinspired trimethoxybenzoylhydrazones: methyl vs nitro groups
Abstract
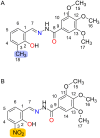
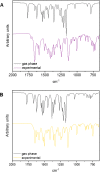
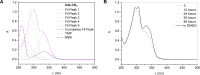
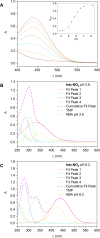
N-Acylhydrazones are a versatile class of organic compounds with a diversity of potential applications. In this study, two new structure-related 3,4,5-trimethoxybenzoyl-containing N-acylhydrazones were synthesized and fully characterized, both in solution and in the solid state. The compounds differ with respect to the carbonyl precursors, i.e., 3-substituted salicylaldehydes with either a methyl or a nitro group. Single crystals of both compounds were isolated from the respective mother liquors and, in both cases, XRD confirmed the obtention of the (E)-isomer, in an anti-conformation. Computational calculations (gas and water phases) were performed in order to confirm some of the structural and vibrational aspects of the compounds. An important intramolecular H bond involving the phenolic hydroxy group and the azomethine nitrogen was identified in the solid state and seems to be maintained in solution. Moreover, the presence of the electron-withdrawing nitro substituent makes this interaction stronger. However, the contact should probably not subsist for the nitro compound under physiological conditions since the presence of this substituent significantly affects the pKa of the phenol: an apparent value of 5.68 ± 0.02 was obtained. This also impacts the basicity of the azomethine nitrogen and, as a consequence, increases the hydrazone's susceptibility to hydrolysis. Nevertheless, both compounds are stable at physiological-like conditions, especially the methyl-derived one, which qualifies them for further toxicological and activity studies, such as those involving trivalent metal ions sequestering in the context of neurodegenerative diseases.
Keywords: DFT calculations; N-acylhydrazones; XRD; phenol acidity; ring substituents.
Copyright © 2023, Martins et al.
Conflict of interest statement
The authors declare no competing interest.
Figures
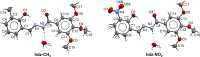

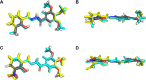


References
-
- Ţînţaş M L, Diac A P, Soran A, Terec A, Grosu I, Bogdan E. J Mol Struct. 2014;1058:106–113. doi: 10.1016/j.molstruc.2013.11.005. - DOI
-
- Wegermann C A, Monzani E, Casella L, Ribeiro M A, Bruzeguini C E T, Vilcachagua J D, Costa L A S, Ferreira A M D C. J Mol Struct. 2022;1250:131633. doi: 10.1016/j.molstruc.2021.131633. - DOI
LinkOut - more resources
Full Text Sources
