Clinical Safety and Efficacy of Pegcetacoplan in a Phase 2 Study of Patients with C3 Glomerulopathy and Other Complement-Mediated Glomerular Diseases
- PMID: 38025230
- PMCID: PMC10658235
- DOI: 10.1016/j.ekir.2023.08.033
Clinical Safety and Efficacy of Pegcetacoplan in a Phase 2 Study of Patients with C3 Glomerulopathy and Other Complement-Mediated Glomerular Diseases
Abstract
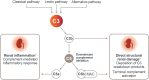
Introduction: Dysregulated complement activation is likely the primary driver of disease in C3 glomerulopathy (C3G) and contributes to other complement-mediated diseases, including immunoglobulin A nephropathy (IgAN), lupus nephritis (LN), and primary membranous nephropathy (PMN). No complement inhibitors are proven to halt disease progression in these diseases. Pegcetacoplan, a targeted C3 and C3b inhibitor, may mitigate complement-mediated kidney damage in C3G and other glomerular diseases in which complement may have a pathogenic role.
Methods: This open-label, phase 2, 48-week study evaluated the preliminary efficacy and safety of subcutaneous pegcetacoplan for patients with complement-mediated glomerular diseases. The primary end point was proteinuria reduction, measured as 24-hour urine protein-to-creatinine ratio. Secondary end points included remission status, changes in estimated glomerular filtration rate (eGFR), and pharmacodynamic biomarkers. Treatment-emergent adverse events (TEAEs) were monitored.
Results: Efficacy results for the C3G cohort are reported herein, along with safety results for the study population. In the C3G cohort, mean proteinuria reduction from baseline to week 48 was 50.9% in the intent-to-treat (ITT) population (n = 7) and 65.4% in the per-protocol (PP) population (n = 4). Mean serum albumin normalized and mean eGFR was stable over 48 weeks. Mean serum C3 levels increased 6-fold and mean soluble C5b-9 levels decreased by 57.3% at week 48. The most common adverse events (AEs) were upper respiratory tract infection, injection site erythema, nausea, and headache. No meningitis or sepsis cases were reported, and no serious treatment-related AEs were observed.
Conclusion: Pegcetacoplan may provide therapeutic benefit for C3G and has a favorable safety profile across the 4 glomerular diseases studied.
Keywords: C3 glomerulopathy; complement; end-stage kidney disease; glomerulonephritis; pegcetacoplan; proteinuria.
© 2023 International Society of Nephrology. Published by Elsevier Inc.
Figures




References
-
- Kazi A.M., Hashmi M.F. StatPearls. StatPearls Publishing LLC; 2022. Glomerulonephritis.https://www.ncbi.nlm.nih.gov/books/NBK560644/
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

