Subthalamic nucleus physiology is correlated with deep brain stimulation motor and non-motor outcomes
- PMID: 38025270
- PMCID: PMC10664412
- DOI: 10.1093/braincomms/fcad268
Subthalamic nucleus physiology is correlated with deep brain stimulation motor and non-motor outcomes
Abstract
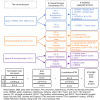
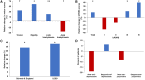
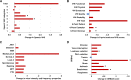
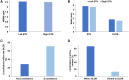
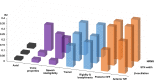
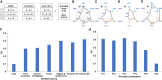
Subthalamic nucleus deep brain stimulation is commonly indicated for symptomatic relief of idiopathic Parkinson's disease. Despite the known improvement in motor scores, affective, cognitive, voice and speech functions might deteriorate following this procedure. Recent studies have correlated motor outcomes with intraoperative microelectrode recordings. However, there are no microelectrode recording-based tools with predictive values relating to long-term outcomes of integrative motor and non-motor symptoms. We conducted a retrospective analysis of the outcomes of patients with idiopathic Parkinson's disease who had subthalamic nucleus deep brain stimulation at Tel Aviv Sourasky Medical Centre (Tel Aviv, Israel) during 2015-2016. Forty-eight patients (19 women, 29 men; mean age, 58 ± 8 years) who were implanted with a subthalamic nucleus deep brain stimulation device underwent pre- and postsurgical assessments of motor, neuropsychological, voice and speech symptoms. Significant improvements in all motor symptoms (except axial signs) and levodopa equivalent daily dose were noted in all patients. Mild improvements were observed in more posterior-related neuropsychological functions (verbal memory, visual memory and organization) while mild deterioration was observed in frontal functions (personality changes, executive functioning and verbal fluency). The concomitant decline in speech intelligibility was mild and only partial, probably in accordance with the neuropsychological verbal fluency results. Acoustic characteristics were the least affected and remained within normal values. Dimensionality reduction of motor, neuropsychological and voice scores rendered six principal components that reflect the main clinical aspects: the tremor-dominant versus the rigidity-bradykinesia-dominant motor symptoms, frontal versus posterior neuropsychological deficits and acoustic characteristics versus speech intelligibility abnormalities. Microelectrode recordings of subthalamic nucleus spiking activity were analysed off-line and correlated with the original scores and with the principal component results. Based on 198 microelectrode recording trajectories, we suggest an intraoperative subthalamic nucleus deep brain stimulation score, which is a simple sum of three microelectrode recording properties: normalized neuronal activity, the subthalamic nucleus width and the relative proportion of the subthalamic nucleus dorsolateral oscillatory region. A threshold subthalamic nucleus deep brain stimulation score >2.5 (preferentially composed of normalized root mean square >1.5, subthalamic nucleus width >3 mm and a dorsolateral oscillatory region/subthalamic nucleus width ratio >1/3) predicts better motor and non-motor long-term outcomes. The algorithm presented here optimizes intraoperative decision-making of deep brain stimulation contact localization based on microelectrode recording with the aim of improving long-term (>1 year) motor, neuropsychological and voice symptoms.
Keywords: Parkinson’s disease; deep brain stimulation; microelectrode recording; subthalamic nucleus; symptoms.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures



References
-
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249(4975):1436–1438. - PubMed
-
- Aziz TZ, Peggs D, Sambrook MA, Crossman AR. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disord. 1991;6(4):288–292. - PubMed
-
- Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349(20):1925–1934. - PubMed
-
- Herzog J, Fietzek U, Hamel W, et al. Most effective stimulation site in subthalamic deep brain stimulation for Parkinson’s disease. Mov Disord. 2004;19(9):1050–1054. - PubMed
LinkOut - more resources
Full Text Sources
