Solid-to-liquid phase transition in the dissolution of cytosolic misfolded-protein aggregates
- PMID: 38025775
- PMCID: PMC10663836
- DOI: 10.1016/j.isci.2023.108334
Solid-to-liquid phase transition in the dissolution of cytosolic misfolded-protein aggregates
Abstract
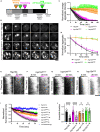
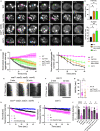
Accumulation of protein aggregates is a hallmark of cellular aging and degenerative disorders. This could result from either increased protein misfolding and aggregation or impaired dissolution of aggregates formed under stress, the latter of which is poorly understood. In this study, we employed quantitative live-cell imaging to investigate the dynamic process of protein disaggregation in yeast. We show that protein aggregates formed upon heat stress are solid condensates, but after stress attenuation these protein aggregates first transition into a liquid-like state during their dissolution. This solid-to-liquid phase transition (SLPT) accompanies the reduction in aggregate number due to the fusion of the liquid condensates. The chaperone activity of Hsp104, a Clp/HSP100 family chaperone, is required for both SLPT and subsequent dispersal of the liquid condensates. Sse1, a yeast HSP110 chaperone, also facilitates SLPT. These results illuminate an unexpected mechanistic framework of cellular control over protein disaggregation upon stress attenuation.
Keywords: Biochemistry; Biological sciences; Cell biology.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

