A 2-year prospective evaluation of airway clearance devices in foreign body airway obstructions
- PMID: 38026136
- PMCID: PMC10658362
- DOI: 10.1016/j.resplu.2023.100496
A 2-year prospective evaluation of airway clearance devices in foreign body airway obstructions
Abstract
Aim: To collect, analyze and report the first prospective, industry-independent, data on airway clearance devices as novel foreign body airway obstruction interventions.
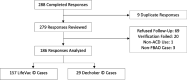
Methods: We recruited adult airway clearance device users between July 1, 2021 and June 30, 2023 using a centralized website and email follow-up. The data collection tool captured patient, responder, situation, and outcome variables. Multi-step respondent validation occurred using electronic and geolocation verification, a random selection follow-up process, and physician review of all submitted cases.
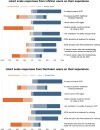
Results: We recruited 186 airway clearance device users (LifeVac©:157 [84.4%]; Dechoker©:29 [15.6%]). LifeVac© was the last intervention before foreign body airway obstruction relief in 151 of 157 cases. Of these, 150 survived to discharge. A basic life support intervention was used before LifeVac© in 119 cases, including the 6 cases where LifeVac© also failed. We identified two adverse events using LifeVac© (perioral bruising), while we could not ascertain whether another 7 were due to the foreign body or LifeVac© (3 = airway edema; 3 = oropharyngeal abrasions; 1 = esophageal perforation). Dechoker© was the last intervention before obstruction relief in 27 of 29 cases and all cases survived. A basic life support intervention was used before Dechoker© in 21 cases, including both where Dechoker© also failed. We identified one adverse event using Dechoker© (oropharyngeal abrasions).
Conclusion: Within these cases, airway clearance devices appear to be effective at relieving foreign body airway obstructions. However, this data should be considered preliminary and hypothesis generating due to several limitations. We urge the resuscitation community to proactively evaluate airway clearance devices to ensure the public remains updated with best practices.
Keywords: ACD; Anti-choking; Basic life support; FBAO; Prehospital; Resuscitation.
© 2023 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors have no competing interests, financial or otherwise, to declare. The research team approached the manufacturers to seek their participation in this study. Manufacturers of airway clearance devices agreed to participate in the study in two areas: identification and recruitment of participants and distributing the research data collection tool as needed. Manufacturers were not involved in study design, nor do they have any financial involvement. Manufacturers do not have access to the collected data, nor have they been permitted to view the results or manuscripts prior to publication. Dr. Amy Peden is funded by a National Health and Medical Research Council (NHMRC) Emerging Leadership Fellowship (Grant ID: APP2009306) which supported open access publication. No funding was obtained for the conduct of the study.
Figures
References
-
- Health Canada . Government of Canada; Ottawa, ON, Canada: 2011. Canadian Injury Data: Mortality 2005 and Hospitalizations, 2001–2005.
-
- Injury Facts. Preventable Death and Death Rates per 100,000 Population in the Home and Community by Cause and Age Group, United States; National Safety Council: Itasca, IL, USA, 2017. Available online: https://injuryfacts.nsc.org/home-and-community/ home-and-community-overv... (accessed on 5 August 2023).
-
- Institute for Health Metrics and Evaluation. Disease and Risk Factor Summaries: Pulmonary Aspiration and Foreign Body in Airway; The Lancet: London, UK, 2019. Available online: https://www.thelancet.com/pb-assets/Lancet/gbd/summaries/diseases/pulmon... (accessed on 5 August 2023).
LinkOut - more resources
Full Text Sources