Derived myeloid lineage induced pluripotent stem as a platform to study human C-C chemokine receptor type 5Δ32 homozygotes
- PMID: 38026202
- PMCID: PMC10663745
- DOI: 10.1016/j.isci.2023.108331
Derived myeloid lineage induced pluripotent stem as a platform to study human C-C chemokine receptor type 5Δ32 homozygotes
Abstract
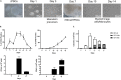
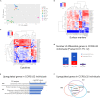
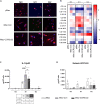
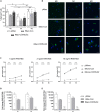
The C-C chemokine receptor type 5 (CCR5) expressed on immune cells supports inflammatory responses by directing cells to the inflammation site. CCR5 is also a major coreceptor for macrophage tropic human immunodeficiency viruses (R5-HIV-1) and its variants can confer protection from HIV infection, making it an ideal candidate to target for therapy. We developed a stepwise protocol that differentiates induced pluripotent stem cells (iPSCs) from individuals homozygous for the CCR5Δ32 variant and healthy volunteers into myeloid lineage induced monocytes (iMono) and macrophages (iMac). By characterizing iMono and iMac against their primary counterparts, we demonstrated that CCR5Δ32 homozygous cells are endowed with similar pluripotent potential for self-renewal and differentiation as iPSC lines generated from non-variant individuals while also showing resistance to HIV infection. In conclusion, these cells are a platform to investigate CCR5 pathophysiology in HIV-positive and negative individuals and to help develop novel therapies.
Keywords: Immunology; Molecular biology; Stem cells research.
Conflict of interest statement
LGB is a member of the Illumina Medical Ethics Committee and receives research funding from Merck, Inc. All the other authors declare no competing interests. The section of “In vitro differentiation of iPSCs in hematopoietic linage cells” described in manuscript is registered under a patent “Human iPSC-derived vascular-related and hematopoietic cells for therapies and toxicology/drug screenings” (patent number #10385313 and 11072778). GC and MB receive royalty income.
Figures




References
-
- Dean M., Carrington M., Winkler C., Huttley G.A., Smith M.W., Allikmets R., Goedert J.J., Buchbinder S.P., Vittinghoff E., Gomperts E., et al. Genetic Restriction of HIV-1 Infection and Progression to AIDS by a Deletion Allele of the CKR5 Structural Gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

