Adverse reactions following COVID-19 vaccine among healthcare professionals working in Ethiopia: a facility-based cross-sectional study
- PMID: 38026432
- PMCID: PMC10654621
- DOI: 10.3389/fpubh.2023.1187948
Adverse reactions following COVID-19 vaccine among healthcare professionals working in Ethiopia: a facility-based cross-sectional study
Abstract
Background of the study: One of the best medical approaches for halting the spread of infectious diseases is vaccination. During the COVID-19 pandemic, healthcare workers (HCWs) were a high-risk population. Due to their susceptibility in terms of their working environment, front-line healthcare personnel should receive vaccinations before others.
Objective: The purpose of this study was to assess the adverse reactions to COVID-19 vaccines among Ethiopian healthcare professionals in 2022.
Methods: A facility-based cross-sectional study design was conducted in Addis Ababa Health Facilities, Ethiopia. A total of 290 health professionals who were vaccinated during the study period were involved. Data entry was done by Epidata (version 3.1) and analyzed using SPSS software version 26. Bivariable analysis was conducted and a p value of less than 0.25 was selected for further multivariable analysis. A p value of 0.05 was considered statistically significant at a 95% confidence level.
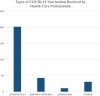
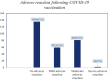
Results: A total of 277 study participants were successfully involved in the study, yielding a response rate of 95.5%. The study participants comprised 123 (44.4%) women and 154 (55.6%) men. The majority of them (202, 72.9%) had received the Oxford AstraZeneca vaccine. Among the 277 study participants, 142 (51.3%) had developed adverse reactions associated with vaccination. Of these, 81 (29.2%) had moderate adverse reactions. Only 2 (0.7%) had developed adverse reactions that led to hospitalization. The most reported short-term adverse reactions were injection site pain (151, 54.5%), headache (114, 41.2%), fever (104, 37.5%), fatigability and tiredness (94, 33.9%), chills (92, 33.2%), muscle pain (79, 28.5%), and decreased sleep quality (34, 12.3%). The multivariable logistic regression showed that the odds of having an adverse reaction were 1.501 times higher among women than men (AOR = 1.501, 95% CI [1.08, 2.754]).
Conclusion and recommendations: This study revealed that adverse effects following the COVID-19 vaccine were moderate in magnitude and minimal in severity. This study showed that adverse reactions that led to hospitalization were rare. Based on the findings of this study, it is recommended that national, multicenter, prospective, and randomized studies be conducted to assess the independent association of each vaccine.
Keywords: COVID-19 vaccine; Oxford AstraZeneca; corona virus; healthcare professionals; vaccine.
Copyright © 2023 Asefa, Derjachew, Belete, Talargia, Melese and Getachew.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ye X, Ye W, Yu J, Gao Y, Ren Z, Chen L, et al. (2021). The landscape of COVID-19 vaccination among healthcare workers at the first round of COVID-19 vaccination in China: willingness, acceptance and self-reported adverse effects. medRxiv [Preprint]. doi: 10.1101/2021.05.15.21257094 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

