The Potential of Novel Synthesized Carbon Dots Derived Resveratrol Using One-Pot Green Method in Accelerating in vivo Wound Healing
- PMID: 38026533
- PMCID: PMC10664763
- DOI: 10.2147/IJN.S434071
The Potential of Novel Synthesized Carbon Dots Derived Resveratrol Using One-Pot Green Method in Accelerating in vivo Wound Healing
Abstract
Background: Carbon dots (CDs), a novel nanomaterial, have gained significant attention over the past decade due to their remarkable fluorescence properties, low toxicity, and biocompatibility. These characteristics make them promising in various applications, especially in biomedicine. However, most CDs are currently synthesized using chemical materials, and their biocompatibility falls short of natural compounds. Research on extracting CDs from natural sources is limited, and their potential in biomedicine remains largely unexplored.
Methods: We extracted CDs from resveratrol, a natural plant compound, and enhanced their water solubility using citric acid. Characterization of resveratrol-based carbon dots (RES-CDs) was carried out using various techniques, including UV-Vis, SEM, TEM, FTIR, XRD, and fluorescence spectroscopy. Extensive biocompatibility tests, wound healing assays, cell migration studies, and angiogenesis experiments were conducted using human umbilical vein endothelial cells (HUVEC). In addition, we investigated the biocompatibility and wound healing potential of RES-CDs in an in vivo rat model of inflammation.
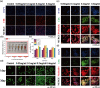
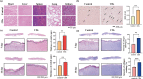
Results: RES-CDs exhibited stable yellow-green fluorescence under 365-nanometer ultraviolet light and demonstrated excellent biocompatibility. In wound healing experiments, RES-CDs outperformed resveratrol in terms of cell scratch healing, migration, and tube formation. In a rat skin defect model, RES-CDs promoted wound healing and stimulated the formation of blood vessels and tissue regeneration near the wound site, as evidenced by increased CD31 and VEGF expression.
Conclusion: Resveratrol-derived CDs with enhanced water solubility show superior performance in tissue healing compared to resveratrol. This discovery opens new possibilities for the clinical application of resveratrol-based carbon dots.
Keywords: bio-imaging; biocompatibility; carbon dots; fluorescence; resveratrol; wound healing.
© 2023 Cheng et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures






References
-
- Neupane GP, Zhang LL, Yildirim T, et al. A prospective future towards bio/medical technology and bioelectronics based on 2D vdWs heterostructures. Nano Res. 2020;13(1):1–17. doi:10.1007/s12274-019-2585-3 - DOI
-
- Ge JX, Zhang QY, Zeng JF, Gu Z, Gao MY. Radiolabeling nanomaterials for multimodality imaging: new insights into nuclear medicine and cancer diagnosis. Biomaterials. 2020;2020:228. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

