Differential metabolic alterations in IDH1 mutant vs. wildtype glioma cells promote epileptogenesis through distinctive mechanisms
- PMID: 38026690
- PMCID: PMC10680369
- DOI: 10.3389/fncel.2023.1288918
Differential metabolic alterations in IDH1 mutant vs. wildtype glioma cells promote epileptogenesis through distinctive mechanisms
Abstract
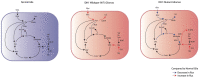
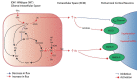
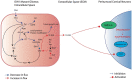
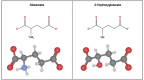
Glioma-related epilepsy (GRE) is a hallmark clinical presentation of gliomas with significant impacts on patient quality of life. The current standard of care for seizure management is comprised of anti-seizure medications (ASMs) and surgical resection. Seizures in glioma patients are often drug-resistant and can often recur after surgery despite total tumor resection. Therefore, current research is focused on the pro-epileptic pathological changes occurring in tumor cells and the peritumoral environment. One important contribution to seizures in GRE patients is metabolic reprogramming in tumor and surrounding cells. This is most evident by the significantly heightened seizure rate in patients with isocitrate dehydrogenase mutated (IDHmut) tumors compared to patients with IDH wildtype (IDHwt) gliomas. To gain further insight into glioma metabolism in epileptogenesis, this review compares the metabolic changes inherent to IDHmut vs. IDHwt tumors and describes the pro-epileptic effects these changes have on both the tumor cells and the peritumoral environment. Understanding alterations in glioma metabolism can help to uncover novel therapeutic interventions for seizure management in GRE patients.
Keywords: brain; glioma; peritumoral excitability; seizure development; tumor-related epilepsy; tumoral metabolism.
Copyright © 2023 McAfee, Moyer, Queen, Mortazavi, Boddeti, Bachani, Zaghloul and Ksendzovsky.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Adhikari S. (2021). “Pathogenesis and management of brain tumor-related epilepsy” in Gliomas. eds. Walker B. C., Mittal S. (Brisbane, AU: Exon Publications; ) - PubMed
-
- Angamo E. A., ul Haq R., Rösner J., Gabriel S., Gerevich Z., Heinemann U., et al. . (2017). Contribution of intrinsic lactate to maintenance of seizure activity in neocortical slices from patients with temporal lobe epilepsy and in rat entorhinal cortex. Int. J. Mol. Sci. 18:1835. doi: 10.3390/ijms18091835, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

