Activity-dependent oligodendrocyte calcium dynamics and their changes in Alzheimer's disease
- PMID: 38026691
- PMCID: PMC10644703
- DOI: 10.3389/fncel.2023.1154196
Activity-dependent oligodendrocyte calcium dynamics and their changes in Alzheimer's disease
Abstract
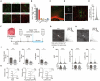
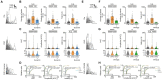
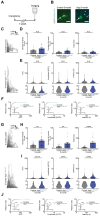
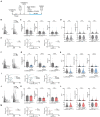
Oligodendrocytes (OCs) form myelin around axons, which is dependent on neuronal activity. This activity-dependent myelination plays a crucial role in training and learning. Previous studies have suggested that neuronal activity regulates proliferation and differentiation of oligodendrocyte precursor cells (OPCs) and myelination. In addition, deficient activity-dependent myelination results in impaired motor learning. However, the functional response of OC responsible for neuronal activity and their pathological changes is not fully elucidated. In this research, we aimed to understand the activity-dependent OC responses and their different properties by observing OCs using in vivo two-photon microscopy. We clarified that the Ca2+ activity in OCs is neuronal activity dependent and differentially regulated by neurotransmitters such as glutamate or adenosine triphosphate (ATP). Furthermore, in 5-month-old mice models of Alzheimer's disease, a period before the appearance of behavioral abnormalities, the elevated Ca2+ responses in OCs are ATP dependent, suggesting that OCs receive ATP from damaged tissue. We anticipate that our research will help in determining the correct therapeutic strategy for neurodegenerative diseases beyond the synapse.
Keywords: ATP; Alzheimer’s disease; glutamate; oligodendrocyte; two photon microscopy.
Copyright © 2023 Yoshida, Kato, Sugio, Takeda and Wake.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

