Neuronal models of TDP-43 proteinopathy display reduced axonal translation, increased oxidative stress, and defective exocytosis
- PMID: 38026702
- PMCID: PMC10679756
- DOI: 10.3389/fncel.2023.1253543
Neuronal models of TDP-43 proteinopathy display reduced axonal translation, increased oxidative stress, and defective exocytosis
Abstract
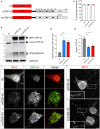
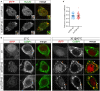
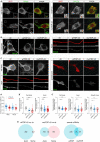
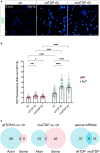
Amyotrophic lateral sclerosis (ALS) is a progressive, lethal neurodegenerative disease mostly affecting people around 50-60 years of age. TDP-43, an RNA-binding protein involved in pre-mRNA splicing and controlling mRNA stability and translation, forms neuronal cytoplasmic inclusions in an overwhelming majority of ALS patients, a phenomenon referred to as TDP-43 proteinopathy. These cytoplasmic aggregates disrupt mRNA transport and localization. The axon, like dendrites, is a site of mRNA translation, permitting the local synthesis of selected proteins. This is especially relevant in upper and lower motor neurons, whose axon spans long distances, likely accentuating their susceptibility to ALS-related noxae. In this work we have generated and characterized two cellular models, consisting of virtually pure populations of primary mouse cortical neurons expressing a human TDP-43 fusion protein, wt or carrying an ALS mutation. Both forms facilitate cytoplasmic aggregate formation, unlike the corresponding native proteins, giving rise to bona fide primary culture models of TDP-43 proteinopathy. Neurons expressing TDP-43 fusion proteins exhibit a global impairment in axonal protein synthesis, an increase in oxidative stress, and defects in presynaptic function and electrical activity. These changes correlate with deregulation of axonal levels of polysome-engaged mRNAs playing relevant roles in the same processes. Our data support the emerging notion that deregulation of mRNA metabolism and of axonal mRNA transport may trigger the dying-back neuropathy that initiates motor neuron degeneration in ALS.
Keywords: TDP-43 proteinopathy; amyotrophic lateral sclerosis; axonal translation; calcium; cortical neurons; oxidative stress; polysome profiling; synaptic function.
Copyright © 2023 Pisciottani, Croci, Lauria, Marullo, Savino, Ambrosi, Podini, Marchioretto, Casoni, Cremona, Taverna, Quattrini, Cioni, Viero, Codazzi and Consalez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Altman T., Ionescu A., Ibraheem A., Priesmann D., Gradus-Pery T., Farberov L., et al. . (2021). Axonal TDP-43 condensates drive neuromuscular junction disruption through inhibition of local synthesis of nuclear encoded mitochondrial proteins. Nat. Commun. 12:6914. doi: 10.1038/s41467-021-27221-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

