Cold physical plasma treatment optimization for improved bone allograft processing
- PMID: 38026873
- PMCID: PMC10661279
- DOI: 10.3389/fbioe.2023.1264409
Cold physical plasma treatment optimization for improved bone allograft processing
Abstract
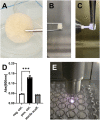
In musculoskeletal surgery, the treatment of large bone defects is challenging and can require the use of bone graft substitutes to restore mechanical stability and promote host-mediated regeneration. The use of bone allografts is well-established in many bone regenerative procedures, but is associated with low rates of ingrowth due to pre-therapeutic graft processing. Cold physical plasma (CPP), a partially ionized gas that simultaneously generates reactive oxygen (O2) and nitrogen (N2) species, is suggested to be advantageous in biomedical implant processing. CPP is a promising tool in allograft processing for improving surface characteristics of bone allografts towards enhanced cellularization and osteoconduction. However, a preclinical assessment regarding the feasibility of pre-therapeutic processing of allogeneic bone grafts with CPP has not yet been performed. Thus, this pilot study aimed to analyze the bone morphology of CPP processed allografts using synchrotron radiation-based microcomputed tomography (SR-µCT) and to analyze the effects of CPP processing on human bone cell viability and function. The analyzes, including co-registration of pre- and post-treatment SR-µCT scans, revealed that the main bone morphological properties (total volume, mineralized volume, surface area, and porosity) remained unaffected by CPP treatment if compared to allografts not treated with CPP. Varying effects on cellular metabolic activity and alkaline phosphatase activity were found in response to different gas mixtures and treatment durations employed for CPP application. It was found that 3 min CPP treatment using a He + 0.1% N2 gas mixture led to the most favourable outcome regarding a significant increase in bone cell viability and alkaline phosphatase activity. This study highlights the promising potential of pre-therapeuthic bone allograft processing by CPP prior to intraoperative application and emphasizes the need for gas source and treatment time optimization for specific applications.
Keywords: allografts; cancellous bone; cold atmospheric pressure plasma; mesenchymal stromal cells; plasma medicine; synchrotron radiation computed tomography.
Copyright © 2023 Fischer, Bortel, Schoon, Behnke, Hesse, Weitkamp, Bekeschus, Pichler, Wassilew and Schulze.
Conflict of interest statement
Authors EmB and BH were employed by Xploraytion GmbH. Author MP was employed by Cells + Tissuebank Austria Gemeinnützige GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Bekeschus S., Schmidt A., Weltmann K. D., von Woedtke T. (2016). The plasma jet kINPen – a powerful tool for wound healing. Clin. Plasma Med. 4 (1), 19–28. 10.1016/j.cpme.2016.01.001 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

