Adverse events associated with molnupiravir: a real-world disproportionality analysis in food and drug administration adverse event reporting system
- PMID: 38026949
- PMCID: PMC10644225
- DOI: 10.3389/fphar.2023.1253799
Adverse events associated with molnupiravir: a real-world disproportionality analysis in food and drug administration adverse event reporting system
Abstract
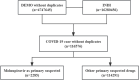
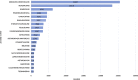
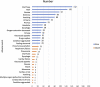
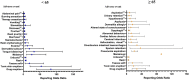
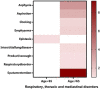
Molnupiravir, an urgently approved drug during the Coronavirus Disease 2019 (COVID-19) pandemic, serves as the basis for our study, which relies on the Food and Drug Administration Adverse Event Reporting System (FAERS). The objective is to extract adverse event (AE) signals associated with molnupiravir from the FAERS database, thereby providing a reference for post-marketing monitoring of adverse events. Specifically, we extracted individual case safety reports (ICSRs) from the database, focusing on cases with COVID-19 indications and molnupiravir identified as the primary suspect drug. Descriptive analysis of the extracted data was performed, followed by four disproportionality analyses using the reporting odds ratio (ROR) method. These analyses were conducted across four levels, encompassing overall data, reports by health professionals, as well as age and gender differentiations, ensuring the robustness of the analysis results. In total, 116,576 ICSRs with COVID-19 indications and 2,285 ICSRs with molnupiravir as the primary suspect were extracted. Notably, after excluding cases with unknown age or gender, a higher proportion of molnupiravir-related ICSRs were observed among individuals aged 65 years and older (70.07%) and women (54.06%). The most frequently reported adverse events and AE signals were associated with gastrointestinal disorders, as well as skin and subcutaneous tissue disorders. Moreover, individuals aged 65 years and older exhibited a higher risk of cardiac disorders, hepatobiliary disorders, renal and urinary disorders, and vascular disorders. In conclusion, this study found molnupiravir demonstrated a lower risk of serious adverse events compared to other RNA antiviral drugs like remdesivir in patients under 65 years old. However, close monitoring of its safety is still necessary for elderly patients aged 65 years and above. Further studies are warranted to continuously assess the safety profile of molnupiravir as its usage increases, especially in high risk populations.
Keywords: adverse events; coronavirus disease 2019; food and drug administration adverse event reporting system; molnupiravir; pharmacovigilance; safety.
Copyright © 2023 Liang, Ma, Wang, Zheng, Su and Lyu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Investigating the Safety Profile of Fast-Track COVID-19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study.Pharmacoepidemiol Drug Saf. 2024 Nov;33(11):e70043. doi: 10.1002/pds.70043. Pharmacoepidemiol Drug Saf. 2024. PMID: 39533148
-
A disproportionality analysis for assessing the safety of FLT3 inhibitors using the FDA Adverse Event Reporting System (FAERS).Ther Adv Drug Saf. 2024 Oct 4;15:20420986241284105. doi: 10.1177/20420986241284105. eCollection 2024. Ther Adv Drug Saf. 2024. PMID: 39381060 Free PMC article.
-
Adverse event profiles of CDK4/6 inhibitors: data mining and disproportionality analysis of the FDA adverse event reporting system.Ther Adv Drug Saf. 2024 Sep 24;15:20420986241278498. doi: 10.1177/20420986241278498. eCollection 2024. Ther Adv Drug Saf. 2024. PMID: 39376495 Free PMC article.
-
Acute renal failure and cardiac arrhythmias associated with remdesivir use in patients with COVID-19 infections: Analysis using the US FDA adverse event reporting system.Int J Risk Saf Med. 2023;34(2):87-99. doi: 10.3233/JRS-220009. Int J Risk Saf Med. 2023. PMID: 37154187
-
Real-world study of adverse events associated with gepant use in migraine treatment based on the VigiAccess and U.S. Food and Drug Administration's adverse event reporting system databases.Front Pharmacol. 2024 Jul 31;15:1431562. doi: 10.3389/fphar.2024.1431562. eCollection 2024. Front Pharmacol. 2024. PMID: 39144633 Free PMC article.
Cited by
-
Benign Pneumatosis Intestinalis in a Patient With Symptomatic COVID-19.Cureus. 2025 Feb 6;17(2):e78604. doi: 10.7759/cureus.78604. eCollection 2025 Feb. Cureus. 2025. PMID: 40062180 Free PMC article.
-
Genetic Predictors of Paxlovid Treatment Response: The Role of IFNAR2, OAS1, OAS3, and ACE2 in COVID-19 Clinical Course.J Pers Med. 2025 Apr 17;15(4):156. doi: 10.3390/jpm15040156. J Pers Med. 2025. PMID: 40278335 Free PMC article.
-
Construction and validation of a cell based reporter assay for identifying inhibitors of SARS coronavirus 2 RNA dependent RNA polymerase activity.Sci Rep. 2025 May 26;15(1):18443. doi: 10.1038/s41598-025-03813-y. Sci Rep. 2025. PMID: 40419748 Free PMC article.
-
Adverse drug events associated with linezolid administration: a real-world pharmacovigilance study from 2004 to 2023 using the FAERS database.Front Pharmacol. 2024 Feb 16;15:1338902. doi: 10.3389/fphar.2024.1338902. eCollection 2024. Front Pharmacol. 2024. PMID: 38434706 Free PMC article.
-
Cost-Effectiveness Analysis of Molnupiravir Versus Best Supportive Care for the Treatment of Outpatient COVID-19 in High-Risk Older Adults in Japan.Pharmacoecon Open. 2025 Jul;9(4):571-584. doi: 10.1007/s41669-025-00578-y. Epub 2025 Apr 23. Pharmacoecon Open. 2025. PMID: 40266489 Free PMC article.
References
-
- Butler C. C., Hobbs F. D. R., Gbinigie O. A., Rahman N. M., Hayward G., Richards D. B., et al. (2023). Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet 401 (10373), 281–293. 10.1016/s0140-6736(22)02597-1 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

