Leydig cell metabolic disorder act as a new mechanism affecting for focal spermatogenesis in Klinefelter syndrome patients: a real world cross-sectional study base on the age
- PMID: 38027184
- PMCID: PMC10650597
- DOI: 10.3389/fendo.2023.1266730
Leydig cell metabolic disorder act as a new mechanism affecting for focal spermatogenesis in Klinefelter syndrome patients: a real world cross-sectional study base on the age
Abstract
Background: Klinefelter's syndrome (KS) was once considered infertile due to congenital chromosomal abnormalities, but the presence of focal spermatozoa changed this. The key to predict and promote spermatogenesis is to find targets that regulate focal spermatogenesis.
Objective: To explore the trend of fertility changes in KS patients at different ages and identify potential therapeutic targets.
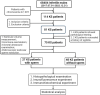
Methods: Bibliometric analysis was used to collect clinical research data on KS from the Web of Science Core Collection (WoSCC) from 1992 to 2022. A cross-sectional study was conducted on 75 KS patients who underwent microscopic testicular sperm extraction (mTESE) from 2017 to 2022 in the real world. The reproductive hormones, testicular histopathology, androgen receptors, insulin-like factor 3 (INSL3) receptors and sperm recovery rate (SRR) were analyzed.
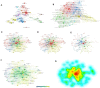
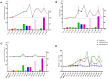
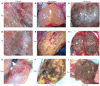
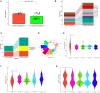
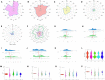
Results: Male infertility, dysplasia, Sertoli cells, Leydig cells, testosterone and spermatogenesis were the research focuses related to KS. Luteinizing hormone (LH), testosterone, and INSL3 were evaluation indicators of Leydig cell function that fluctuate with age. Testosterone and LH peaked at ages 13-19 and 30-45, while INSL3 only peaked at ages 13-19. 27 patients (27/75) recovered sperm through mTESE and experienced SRR peaks at the ages of 20, 28, 34, and 37. The SRR of fibrosis patients was 46.15%, fatty degeneration was 7.14%, and melanosis was 40.00%. The INSL3 and androgen receptors were highly expressed and roughly balanced in focal spermatogenesis.
Conclusion: Abnormal metabolism of Leydig cells led to imbalanced expression of INSL3 and androgen receptors, which might be a potential target for spermatogenesis in KS.
Keywords: Klinefelter syndrome; Leydig cell; age; metabolic disorder; microscopic testicular sperm extraction; spermatogenic.
Copyright © 2023 Liu, Zhang, Gao, Lin, Zhu, Zheng, Ye, Luo, Qing, Xiao, Hu, Zhou and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Klinefelter HF, Reifenstein EC, Albright F. Syndrome characterized by gynecomastia, aspermatogenesis without A-leydigism, and increased excretion of follicle-stimulating hormone. J Clin Endocrinol (1942) 2(11):615–27. doi: 10.1210/jcem-2-11-615 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

