T cell immune memory after covid-19 and vaccination
- PMID: 38027416
- PMCID: PMC10668147
- DOI: 10.1136/bmjmed-2022-000468
T cell immune memory after covid-19 and vaccination
Abstract
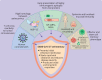
The T cell memory response is a crucial component of adaptive immunity responsible for limiting or preventing viral reinfection. T cell memory after infection with the SARS-CoV-2 virus or vaccination is broad, and spans multiple viral proteins and epitopes, about 20 in each individual. So far the T cell memory response is long lasting and provides a high level of cross reactivity and hence resistance to viral escape by variants of the SARS-CoV-2 virus, such as the omicron variant. All current vaccine regimens tested produce robust T cell memory responses, and heterologous regimens will probably enhance protective responses through increased breadth. T cell memory could have a major role in protecting against severe covid-19 disease through rapid viral clearance and early presentation of epitopes, and the presence of cross reactive T cells might enhance this protection. T cell memory is likely to provide ongoing protection against admission to hospital and death, and the development of a pan-coronovirus vaccine might future proof against new pandemic strains.
Keywords: COVID-19; Covid-19; Immunology; Respiratory tract infections.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: CJAD has done consultancy for Synairgen and Moderna, has been an investigator on commercial SARS-CoV-2 vaccine studies (AstraZeneca, Moderna, and Valneva), receives current research funding from UK MRC, and previous funding from DHSC, Wellcome, and Barbour Foundation; LT has received consulting fees from MHRA, and from AstraZeneca and Synairgen, paid to the University of Liverpool, speakers’ fees from Eisai Ltd, and support for conference attendance from AstraZeneca. PK has done consultancy work for Astra Zeneca, UCB, MedImmuneBio, GlaxoSimthKline, Ysios, Biomunex, and Infinitopes, has received competitive grant funding for basic science investigations of inflammatory bowel disease from Pfizer and Johnson&Jonhson through external grant award schemes, and has been granted a patent to generate anticancer vaccines using adenovirus vectors with CRUK; SJD is a member of the UK government’s New and Emerging Respiratory Virus Threats Advisory Group; AR is co-investigator in a phase III study evaluation of a monoclonal antibody for pre-exposure prophylaxis with GlaxoSmithKline and received compensation from Takaeda and CSL Biotechnology for conference attendances invited talks.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
