Formation of amyloid fibrils from ovalbumin under Ohmic heating
- PMID: 38027889
- PMCID: PMC10658388
- DOI: 10.1016/j.heliyon.2023.e22061
Formation of amyloid fibrils from ovalbumin under Ohmic heating
Abstract
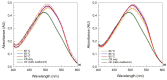
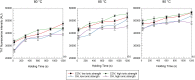
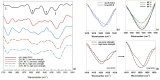
Ohmic heating (OH) is an alternative sustainable heating technology that has demonstrated its potential to modify protein structures and aggregates. Furthermore, certain protein aggregates, namely amyloid fibrils (AF), are associated with an enhanced protein functionality, such as gelation. This study evaluates how Ohmic heating (OH) influences the formation of AF structures from ovalbumin source under two electric field strength levels, 8.5 to 10.5 and 24.0-31.0 V/cm, respectively. Hence, AF aggregate formation was assessed over holding times ranging from 30 to 1200 sunder various environmental conditions (3.45 and 67.95 mM NaCl, 80, 85 and 90 °C, pH = 7). AF were formed under all conditions. SDS-PAGE revealed that OH had a higher tendency to preserve native ovalbumin molecules. Furthermore, Congo Red and Thioflavin T stainings indicated that OH reduces the amount of AF structures. This finding was supported by FTIR measurements, which showed OH samples to contain lower amounts of beta-sheets. Field flow fractioning revealed smaller-sized aggregates or aggregate clusters occurred after OH treatment. In contrast, prolonged holding time or higher treatment temperatures increased ThT fluorescence, beta-sheet structures and aggregate as well as cluster sizes. Ionic strength was found to dominate the effects of electric field strength under different environmental conditions.
Keywords: Amyloid fibril formation; Cross-beta sheet structures; Field flow fractioning; Moderate electric fields; Protein aggregation; Thioflavin T.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Goullieux A., Pain J.-P. Chapter 22 – Ohmic Heating. second ed. Elsevier Ltd; 2014. - DOI
-
- Fellows P.J. 2017. Dielectric, Ohmic and Infrared Heating. - DOI
-
- Jaeger H., Roth A., Toepfl S., Holzhauser T., Engel K.H., Knorr D., Vogel R.F., Bandick N., Kulling S., Heinz V., Steinberg P. Opinion on the use of ohmic heating for the treatment of foods. Trends Food Sci. Technol. 2016;55:84–97. doi: 10.1016/j.tifs.2016.07.007. - DOI
LinkOut - more resources
Full Text Sources

