The microtubule inhibitor eribulin demonstrates efficacy in platinum-resistant and refractory high-grade serous ovarian cancer patient-derived xenograft models
- PMID: 38028140
- PMCID: PMC10666702
- DOI: 10.1177/17588359231208674
The microtubule inhibitor eribulin demonstrates efficacy in platinum-resistant and refractory high-grade serous ovarian cancer patient-derived xenograft models
Abstract
Background: Despite initial response to platinum-based chemotherapy and PARP inhibitor therapy (PARPi), nearly all recurrent high-grade serous ovarian cancer (HGSC) will acquire lethal drug resistance; indeed, ~15% of individuals have de novo platinum-refractory disease.
Objectives: To determine the potential of anti-microtubule agent (AMA) therapy (paclitaxel, vinorelbine and eribulin) in platinum-resistant or refractory (PRR) HGSC by assessing response in patient-derived xenograft (PDX) models of HGSC.
Design and methods: Of 13 PRR HGSC PDX, six were primary PRR, derived from chemotherapy-naïve samples (one was BRCA2 mutant) and seven were from samples obtained following chemotherapy treatment in the clinic (five were mutant for either BRCA1 or BRCA2 (BRCA1/2), four with prior PARPi exposure), recapitulating the population of individuals with aggressive treatment-resistant HGSC in the clinic. Molecular analyses and in vivo treatment studies were undertaken.
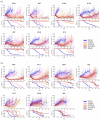
Results: Seven out of thirteen PRR PDX (54%) were sensitive to treatment with the AMA, eribulin (time to progressive disease (PD) ⩾100 days from the start of treatment) and 11 out of 13 PDX (85%) derived significant benefit from eribulin [time to harvest (TTH) for each PDX with p < 0.002]. In 5 out of 10 platinum-refractory HGSC PDX (50%) and one out of three platinum-resistant PDX (33%), eribulin was more efficacious than was cisplatin, with longer time to PD and significantly extended TTH (each PDX p < 0.02). Furthermore, four of these models were extremely sensitive to all three AMA tested, maintaining response until the end of the experiment (120d post-treatment start). Despite harbouring secondary BRCA2 mutations, two BRCA2-mutant PDX models derived from heavily pre-treated individuals were sensitive to AMA. PRR HGSC PDX models showing greater sensitivity to AMA had high proliferative indices and oncogene expression. Two PDX models, both with prior chemotherapy and/or PARPi exposure, were refractory to all AMA, one of which harboured the SLC25A40-ABCB1 fusion, known to upregulate drug efflux via MDR1.
Conclusion: The efficacy observed for eribulin in PRR HGSC PDX was similar to that observed for paclitaxel, which transformed ovarian cancer clinical practice. Eribulin is therefore worthy of further consideration in clinical trials, particularly in ovarian carcinoma with early failure of carboplatin/paclitaxel chemotherapy.
Keywords: anti-microtubule agent; eribulin; high-grade serous ovarian cancer; homologous recombination deficiency; paclitaxel; platinum resistance; proliferative index.
© The Author(s), 2023.
Conflict of interest statement
Eisai Inc. provided drug support for this study. DDB declares Consultant for Exo Therapeutics. CLS declares Advisory Boards for AstraZeneca, Clovis Oncology, Roche, Eisai Inc., Sierra Oncology, Takeda, MSD, EpsilaBio and Grant/Research support from Clovis Oncology, Eisai Inc., Sierra Oncology, Roche, Beigene, AstraZeneca and Boehringer Ingelheim. EMS declares Scientific Advisory Board for Ideaya Biosciences and DSMB role for Novartis. Other authors declare no conflicts of interest.
Figures


References
-
- Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol 2003; 21: 3194–3200. - PubMed
-
- Piccart MJ, Bertelsen K, James K, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst 2000; 92: 699–708. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

