Zoledronic acid sequential to teriparatide may promote greater inhibition of bone resorption than zoledronic acid alone
- PMID: 38028331
- PMCID: PMC10666713
- DOI: 10.1177/20420188231213639
Zoledronic acid sequential to teriparatide may promote greater inhibition of bone resorption than zoledronic acid alone
Abstract
Background: Teriparatide (TPTD) should be followed by an antiresorptive to maximize bone mineral density gain and anti-fracture protection. Infrequent zoledronic acid (ZOL) administration has demonstrated effectiveness. The duration of ZOL effect following TPTD is unknown.
Objective: To evaluate the effect of ZOL on bone resorption marker in a post-TPTD versus ZOL-alone scenario in osteoporotic patients.
Design: Retrospective cohort study.
Methods: Patients treated with TPTD followed by ZOL (TPTD-ZOL) or with a single ZOL infusion were identified in the database of a tertiary referral center. Clinical and laboratory data, including C-terminal telopeptide of type I collagen (CTX) following ZOL treatment, were compared.
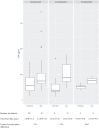
Results: Twenty-six patients (93% women) treated with TPTD-ZOL and 41 with ZOL were comparable in age (median 70.1 versus 69.6 years, p = 0.6) and sex. Timing of CTX measurement post-ZOL was the same, median 1.0 year. CTX was lower following TPTD-ZOL (median 142.1 versus 184.2 pg/mL, p = 0.005). In a multivariable regression model (controlled for baseline characteristics), pretreatment with TPTD strongly predicted CTX <150 pg/mL, 1 year following ZOL (odds ratio = 7.5, 95% CI 1.3-58.1, p = 0.03). In a subgroup with sequential CTX measurements following one ZOL, significantly lower levels persisted in the TPTD-ZOL group for a median of 4.4 years follow-up.
Conclusion: ZOL-administered sequential to TPTD yielded deeper and more prolonged bone resorption suppression than ZOL alone. Prospective data are needed to confirm whether in a sequential treatment scenario, subsequent ZOL dosing interval should be less frequent.
Keywords: anabolic therapy; bisphosphonates; bone resorption; osteoporosis.
© The Author(s), 2023.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
-
- Langdahl B. Treatment of postmenopausal osteoporosis with bone-forming and antiresorptive treatments: combined and sequential approaches. Bone 2020; 139: 115516. - PubMed
-
- Eastell R, Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol 2017; 5: 908–923. - PubMed
-
- Lindsay R, Scheele WH, Neer R, et al. Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med 2004; 164: 2024–2030. - PubMed
-
- Muschitz C, Kocijan R, Fahrleitner-Pammer A, et al. Overlapping and continued alendronate or raloxifene administration in patients on teriparatide: effects on areal and volumetric bone mineral density – the CONFORS study. J Bone Miner Res 2014; 29: 1777–1785. - PubMed
LinkOut - more resources
Full Text Sources