Revolutionizing orofacial pain management: the promising potential of stem cell therapy
- PMID: 38028430
- PMCID: PMC10679438
- DOI: 10.3389/fpain.2023.1239633
Revolutionizing orofacial pain management: the promising potential of stem cell therapy
Abstract
Orofacial pain remains a significant health issue in the United States. Pain originating from the orofacial region can be composed of a complex array of unique target tissue that contributes to the varying success of pain management. Long-term use of analgesic drugs includes adverse effects such as physical dependence, gastrointestinal bleeding, and incomplete efficacy. The use of mesenchymal stem cells for their pain relieving properties has garnered increased attention. In addition to the preclinical and clinical results showing stem cell analgesia in non-orofacial pain, studies have also shown promising results for orofacial pain treatment. Here we discuss the outcomes of mesenchymal stem cell treatment for pain and compare the properties of stem cells from different tissues of origin. We also discuss the mechanism underlying these analgesic/anti-nociceptive properties, including the role of immune cells and the endogenous opioid system. Lastly, advancements in the methods and procedures to treat patients experiencing orofacial pain with mesenchymal stem cells are also discussed.
Keywords: MSC secretome; mechanisms of stem cell anti-nociception; orofacial pain; stem cell analgesia; trigeminal pain.
© 2023 Ren, Vickers, Murillo and Ruparel.
Conflict of interest statement
RV declares a conflict of interest as he is the Director and surgeon involved in treating patients with stem cell treatments for Clinical Stem Cells Pty Ltd. (Australia). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

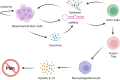


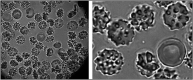

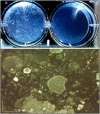
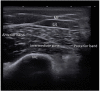

References
-
- Black LL, Gaynor J, Gahring D, Adams C, Aron D, Harman S, et al. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther. (2007) 8(4):272–84. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

