Antiviral activity of nitazoxanide against Morbillivirus infections
- PMID: 38028567
- PMCID: PMC10679774
- DOI: 10.1016/j.jve.2023.100353
Antiviral activity of nitazoxanide against Morbillivirus infections
Abstract
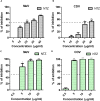
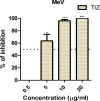
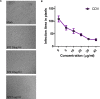
The measles virus (MeV) and canine distemper virus (CDV) belong to the genus Morbillivirus of the Paramyxoviridae family. They are enveloped viruses harboring a non-segmented negative-sense RNA. Morbilliviruses are extremely contagious and transmitted through infectious aerosol droplets. Both MeV and CDV may cause respiratory infections and fatal encephalitis, although a high incidence of brain infections is unique to CDV. Despite the availability of a safe and effective vaccine against these viruses, in recent years we are witnessing a strong resurgence of Morbillivirus infection. Measles still kills more than 100,000 people each year, and CDV causes widespread outbreaks, especially among wild animals, including non-human primates. No drugs are currently approved for MeV and CDV. Therefore, the identification of effective antiviral agents represents an unmet medical need. Here, we have investigated the potential antiviral properties of nitazoxanide (NTZ) against MeV and CDV. Antiviral activity was explored with live virus and cell-based assays. NTZ is a thiazolide that is approved by the FDA as an antiprotozoal agent for the treatment of Giardia intestinalis and Cryptosporidium parvum. Further, nitazoxanide and its metabolite tizoxanide have recently emerged as broad-spectrum antiviral agents. We found that NTZ blocks the MeV and CDV replication, acting at the post-entry level. Moreover, we showed that NTZ affects the function of the viral fusion protein (F), impairing viral spread. Our results indicate that NTZ should be further explored as a therapeutic option in measles and canine distemper virus treatment.
Keywords: Antiviral; Canine distemper virus; Measles; Morbillivirus; Nitazoxanide; Thiazolide.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Rational attenuation of canine distemper virus (CDV) to develop a morbillivirus animal model that mimics measles in humans.J Virol. 2024 Mar 19;98(3):e0185023. doi: 10.1128/jvi.01850-23. Epub 2024 Feb 28. J Virol. 2024. PMID: 38415596 Free PMC article.
-
Biological properties of phocine distemper virus and canine distemper virus.APMIS Suppl. 1993;36:1-51. APMIS Suppl. 1993. PMID: 8268007 Review.
-
Canine and Phocine Distemper Viruses: Global Spread and Genetic Basis of Jumping Species Barriers.Viruses. 2019 Oct 14;11(10):944. doi: 10.3390/v11100944. Viruses. 2019. PMID: 31615092 Free PMC article. Review.
-
Canine Distemper Virus Pathogenesis in the Ferret Model.Methods Mol Biol. 2024;2808:197-208. doi: 10.1007/978-1-0716-3870-5_15. Methods Mol Biol. 2024. PMID: 38743372
-
Antiviral efficacy of favipiravir against canine distemper virus infection in vitro.BMC Vet Res. 2019 Sep 2;15(1):316. doi: 10.1186/s12917-019-2057-8. BMC Vet Res. 2019. PMID: 31477101 Free PMC article.
References
-
- Sykes J.E. Small Animal Critical Care Medicine. Elsevier; 2015. Viral infections.https://linkinghub.elsevier.com/retrieve/pii/B9781455703067000969 [Internet] [cited 2022 Oct 12]. pp. 504–8. Available from:
-
- Paramyxoviridae and Pneumoviridae . Elsevier; 2017. Fenner's Veterinary Virology.https://linkinghub.elsevier.com/retrieve/pii/B9780128009468000179 [Internet] [cited 2022 Oct 12]. pp. 327–56. Available from:
LinkOut - more resources
Full Text Sources

