Next generation multiplexing for digital PCR using a novel melt-based hairpin probe design
- PMID: 38028620
- PMCID: PMC10667681
- DOI: 10.3389/fgene.2023.1272964
Next generation multiplexing for digital PCR using a novel melt-based hairpin probe design
Abstract
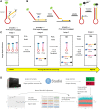
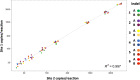
Digital PCR (dPCR) is a powerful tool for research and diagnostic applications that require absolute quantification of target molecules or detection of rare events, but the number of nucleic acid targets that can be distinguished within an assay has limited its usefulness. For most dPCR systems, one target is detected per optical channel and the total number of targets is limited by the number of optical channels on the platform. Higher-order multiplexing has the potential to dramatically increase the usefulness of dPCR, especially in scenarios with limited sample. Other potential benefits of multiplexing include lower cost, additional information generated by more probes, and higher throughput. To address this unmet need, we developed a novel melt-based hairpin probe design to provide a robust option for multiplexing digital PCR. A prototype multiplex digital PCR (mdPCR) assay using three melt-based hairpin probes per optical channel in a 16-well microfluidic digital PCR platform accurately distinguished and quantified 12 nucleic acid targets per well. For samples with 10,000 human genome equivalents, the probe-specific ranges for limit of blank were 0.00%-0.13%, and those for analytical limit of detection were 0.00%-0.20%. Inter-laboratory reproducibility was excellent (r 2 = 0.997). Importantly, this novel melt-based hairpin probe design has potential to achieve multiplexing beyond the 12 targets/well of this prototype assay. This easy-to-use mdPCR technology with excellent performance characteristics has the potential to revolutionize the use of digital PCR in research and diagnostic settings.
Keywords: absolute quantification; digital PCR; higher-order multiplexing; mdPCR assay; melt-based hairpin probe design.
Copyright © 2023 Edwards, Takach, McAndrew, Menteer, Lestz, Whitman and Baxter-Lowe.
Conflict of interest statement
JT, MM, and DW are employed by Luminex Corporation (A Diasorin Company), Austin, TX. DW and Luminex Corporation holds a patent related to this work. DW has stock ownership in Diasorin. LAB-L is a member of the Transplant Advisory board at Luminex Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Clausen F. B., Jorgensen K., Wardil L. W., Nielsen L. K., Krog G. R. (2023). Droplet digital PCR-based testing for donor-derived cell-free DNA in transplanted patients as noninvasive marker of allograft health: methodological aspects. PLoS One 18 (2), e0282332. 10.1371/journal.pone.0282332 - DOI - PMC - PubMed

