Effect of ethylene pretreatment on tomato plant responses to salt, drought, and waterlogging stress
- PMID: 38028648
- PMCID: PMC10654692
- DOI: 10.1002/pld3.548
Effect of ethylene pretreatment on tomato plant responses to salt, drought, and waterlogging stress
Abstract
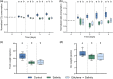
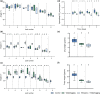
Salinity, drought, and waterlogging are common environmental stresses that negatively impact plant growth, development, and productivity. One of the responses to abiotic stresses is the production of the phytohormone ethylene, which induces different coping mechanisms that help plants resist or tolerate stress. In this study, we investigated if an ethylene pretreatment can aid plants in activating stress-coping responses prior to the onset of salt, drought, and waterlogging stress. Therefore, we measured real-time transpiration and CO2 assimilation rates and the impact on biomass during and after 3 days of abiotic stress. Our results showed that an ethylene pretreatment of 1 ppm for 4 h did not significantly influence the negative effects of waterlogging stress, while plants were more sensitive to salt stress as reflected by enhanced water losses due to a higher transpiration rate. However, when exposed to drought stress, an ethylene pretreatment resulted in reduced transpiration rates, reducing water loss during drought stress. Overall, our findings indicate that pretreating tomato plants with ethylene can potentially regulate their responses during the forthcoming stress period, but optimization of the ethylene pre-treatment duration, timing, and dose is needed. Furthermore, it remains tested if the effect is related to the stress duration and severity and whether an ethylene pretreatment has a net positive or negative effect on plant vigor during stress recovery. Further investigations are needed to elucidate the mode of action of how ethylene priming impacts subsequent stress responses.
Keywords: abiotic stress; drought; ethylene; hypoxia; priming; salt stress; waterlogging.
© 2023 The Authors. Plant Direct published by American Society of Plant Biologists and the Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Bashar, K. K. , Tareq, M. Z. , Amin, M. R. , Honi, U. , Tahjib‐Ul‐Arif, M. , Sadat, M. A. , & Hossen, Q. M. M. (2019). Phytohormone‐mediated stomatal response, escape and quiescence strategies in plants under flooding stress. Agronomy, 9(2), 43. 10.3390/agronomy9020043 - DOI
-
- Borbély, P. , Poór, P. , & Tari, I. (2020). Changes in physiological and photosynthetic parameters in tomato of different ethylene status under salt stress: Effects of exogenous 1‐aminocyclopropane‐1‐carboxylic acid treatment and the inhibition of ethylene signalling. Plant Physiology and Biochemistry, 156(September), 345–356. 10.1016/j.plaphy.2020.09.019 - DOI - PubMed
LinkOut - more resources
Full Text Sources

