Metabolic Control of Cardiomyocyte Cell Cycle
- PMID: 38028975
- PMCID: PMC10655756
- DOI: 10.14797/mdcvj.1309
Metabolic Control of Cardiomyocyte Cell Cycle
Abstract
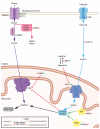
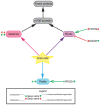
Current therapies for heart failure aim to prevent the deleterious remodeling that occurs after MI injury, but currently no therapies are available to replace lost cardiomyocytes. Several organisms now being studied are capable of regenerating their myocardium by the proliferation of existing cardiomyocytes. In this review, we summarize the main metabolic pathways of the mammalian heart and how modulation of these metabolic pathways through genetic and pharmacological approaches influences cardiomyocyte proliferation and heart regeneration.
Keywords: cardiac regeneration; cardiomyocyte proliferation; glycolysis; oxidative phosphorylation; reactive oxygen species; uridine diphosphate N-acetylglucosamine (UDP GlycNAC).
Copyright: © 2023 The Author(s).
Conflict of interest statement
HAS is supported by NIH R01 HL149137-01, NIH 1P01HL160476-01A1, NIH R35 HL166563-01, and NIH P01HL160488. IM-M is supported by AHA Postdoctoral Fellowship 903385.The authors have no competing interests to declare.
Figures

References
-
- Makinde AO, Kantor PF, Lopaschuk GD. Maturation of fatty acid and carbohydrate metabolism in the newborn heart. Mol Cell Biochem. 1998. Nov;188(1-2):49-56. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

