Whole genome analysis of Rhizopus species causing rhino-cerebral mucormycosis during the COVID-19 pandemic
- PMID: 38029246
- PMCID: PMC10644343
- DOI: 10.3389/fcimb.2023.1251456
Whole genome analysis of Rhizopus species causing rhino-cerebral mucormycosis during the COVID-19 pandemic
Abstract
Introduction: Mucormycosis is an acute invasive fungal disease (IFD) seen mainly in immunocompromised hosts and in patients with uncontrolled diabetes. The incidence of mucormycosis increased exponentially in India during the SARS-CoV-2 (henceforth COVID-19) pandemic. Since there was a lack of data on molecular epidemiology of Mucorales causing IFD during and after the COVID-19 pandemic, whole genome analysis of the Rhizopus spp. isolated during this period was studied along with the detection of mutations that are associated with antifungal drug resistance.
Materials and methods: A total of 50 isolates of Rhizopus spp. were included in this prospective study, which included 28 from patients with active COVID-19 disease, 9 from patients during the recovery phase, and 13 isolates from COVID-19-negative patients. Whole genome sequencing (WGS) was performed for the isolates, and the de novo assembly was done with the Spades assembler. Species identification was done by extracting the ITS gene sequence from each isolate followed by searching Nucleotide BLAST. The phylogenetic trees were made with extracted ITS gene sequences and 12 eukaryotic core marker gene sequences, respectively, to assess the genetic distance between our isolates. Mutations associated with intrinsic drug resistance to fluconazole and voriconazole were analyzed.
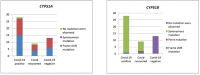
Results: All 50 patients presented to the hospital with acute fungal rhinosinusitis. These patients had a mean HbA1c of 11.2%, and a serum ferritin of 546.8 ng/mL. Twenty-five patients had received steroids. By WGS analysis, 62% of the Rhizopus species were identified as R. delemar. Bayesian analysis of population structure (BAPS) clustering categorized these isolates into five different groups, of which 28 belong to group 3, 9 to group 5, and 8 to group 1. Mutational analysis revealed that in the CYP51A gene, 50% of our isolates had frameshift mutations along with 7 synonymous mutations and 46% had only synonymous mutations, whereas in the CYP51B gene, 68% had only synonymous mutations and 26% did not have any mutations.
Conclusion: WGS analysis of Mucorales identified during and after the COVID-19 pandemic gives insight into the molecular epidemiology of these isolates in our community and establishes newer mechanisms for intrinsic azole resistance.
Keywords: COVID - 19; Mucorales; azole resistance detection; molecular epidemiology; whole genome sequencing.
Copyright © 2023 Michael, Venkatesan, Ninan, Solaimalai, Sumanth, Varghese, Kurien, Varghese and C.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aranjani J. M., Manuel A., Razack H. I. A., Mathew S. T. (2021). COVID-19–associated mucormycosis: Evidence-based critical review of an emerging infection burden during the pandemic’s second wave in India. PloS Negl. Trop. Dis. 15 (11), e0009921. doi: 10.1371/journal.pntd.0009921 - DOI - PMC - PubMed
-
- Cendejas-Bueno E., Kolecka A., Alastruey-Izquierdo A., Theelen B., Groenewald M., Kostrzewa M., et al. (2012). Reclassification of the Candida haemulonii complex as Candida haemulonii (C. haemulonii group I), C. duobushaemulonii sp. nov. (C. haemulonii group II) and C. haemulonii var. vulnera var. nov.: three multiresistant human pathogenic yeasts. J. Clin. Microbiol. 50 (11), 3641–3651. doi: 10.1128/JCM.02248-12 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous