Comparative proteome analysis revealed the differences in response to both Mycobacterium tuberculosis and Mycobacterium bovis infection of bovine alveolar macrophages
- PMID: 38029268
- PMCID: PMC10646506
- DOI: 10.3389/fcimb.2023.1266884
Comparative proteome analysis revealed the differences in response to both Mycobacterium tuberculosis and Mycobacterium bovis infection of bovine alveolar macrophages
Abstract
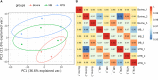
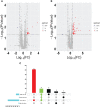
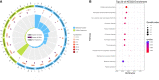
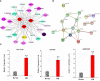
Tuberculosis (TB), attributed to the Mycobacterium tuberculosis complex, is one of the most serious zoonotic diseases worldwide. Nevertheless, the host mechanisms preferentially leveraged by Mycobacterium remain unclear. After infection, both Mycobacterium tuberculosis (MTB) and Mycobacterium bovis (MB) bacteria exhibit intimate interactions with host alveolar macrophages; however, the specific mechanisms underlying these macrophage responses remain ambiguous. In our study, we performed a comparative proteomic analysis of bovine alveolar macrophages (BAMs) infected with MTB or MB to elucidate the differential responses of BAMs to each pathogen at the protein level. Our findings revealed heightened TB infection susceptibility of BAMs that had been previously infected with MTB or MB. Moreover, we observed that both types of mycobacteria triggered significant changes in BAM energy metabolism. A variety of proteins and signalling pathways associated with autophagy and inflammation-related progression were highly activated in BAMs following MB infection. Additionally, proteins linked to energy metabolism were highly expressed in BAMs following MTB infection. In summary, we propose that BAMs may resist MTB and MB infections via different mechanisms. Our findings provide critical insights into TB pathogenesis, unveiling potential biomarkers to facilitate more effective TB treatment strategies. Additionally, our data lend support to the hypothesis that MTB may be transmitted via cross-species infection.
Keywords: Mycobacterium tuberculosis; autophagy; bovine tuberculosis; inflammatory response; resistance mechanism.
Copyright © 2023 Cai, Gao, Wang, Zhang, Wang, Jiang, Zeng, Wang, Wu and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Athman J. J., Wang Y., McDonald D. J., Boom W. H., Harding C. V., Wearsch P. A.. (2015). Bacterial membrane vesicles mediate the release of mycobacterium tuberculosis lipoglycans and lipoproteins from infected macrophages. J. Immunol. (Baltimore Md. 1950) 195 (3), 1044–1053. doi: 10.4049/jimmunol.1402894 - DOI - PMC - PubMed
-
- Blázquez J., Espinosa de Los Monteros L. E., Samper S., Martín C., Guerrero A., Cobo J., et al. . (1997). Genetic characterization of multidrug-resistant Mycobacterium bovis strains from a hospital outbreak involving human immunodeficiency virus-positive patients. J. Clin. Microbiol. 35 (6), 1390–1393. doi: 10.1128/jcm.35.6.1390-1393.1997 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

