A direct interaction between CPF and RNA Pol II links RNA 3' end processing to transcription
- PMID: 38029752
- PMCID: PMC10783616
- DOI: 10.1016/j.molcel.2023.11.004
A direct interaction between CPF and RNA Pol II links RNA 3' end processing to transcription
Abstract
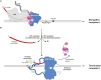
Transcription termination by RNA polymerase II (RNA Pol II) is linked to RNA 3' end processing by the cleavage and polyadenylation factor (CPF or CPSF). CPF contains endonuclease, poly(A) polymerase, and protein phosphatase activities, which cleave and polyadenylate pre-mRNAs and dephosphorylate RNA Pol II to control transcription. Exactly how the RNA 3' end processing machinery is coupled to transcription remains unclear. Here, we combine in vitro reconstitution, structural studies, and genome-wide analyses to show that yeast CPF physically and functionally interacts with RNA Pol II. Surprisingly, CPF-mediated dephosphorylation promotes the formation of an RNA Pol II stalk-to-stalk homodimer in vitro. This dimer is compatible with transcription but not with the binding of transcription elongation factors. Disruption of the dimerization interface in cells causes transcription defects, including altered RNA Pol II abundance on protein-coding genes, tRNA genes, and intergenic regions. We hypothesize that RNA Pol II dimerization may provide a mechanistic basis for the allosteric model of transcription termination.
Keywords: CPSF; X-ray crystallography; cryo-EM; phosphatase; poly(A) tail; polymerase; transcription.
Copyright © 2023 MRC Laboratory of Molecular Biology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.A.P. is an inventor of a patent filed by the Medical Research Council for all-gold EM supports, licensed to Quantifoil under the trademark UltrAuFoil, and is on the advisory board of Molecular Cell.
Figures







References
-
- Hirose Y., Manley J.L. RNA polymerase II is an essential mRNA polyadenylation factor. Nature. 1998;395:93–96. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

