Comparison of Pirfenidone and Nintedanib: Post Hoc Analysis of the CleanUP-IPF Study
- PMID: 38030064
- PMCID: PMC11110676
- DOI: 10.1016/j.chest.2023.11.035
Comparison of Pirfenidone and Nintedanib: Post Hoc Analysis of the CleanUP-IPF Study
Abstract
Background: Antifibrotics are effective in slowing FVC decline in idiopathic pulmonary fibrosis (IPF). However, whether antifibrotic type is differentially associated with FVC decline remains inconclusive.
Research question: Are there significant differences in 12-month FVC decline between pirfenidone and nintedanib?
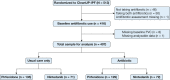
Study design and methods: A post hoc analysis was performed using the Clinical Efficacy of Antimicrobial Therapy Strategy Using Pragmatic Design in IPF (CleanUP-IPF) trial (No. NCT02759120). Participants who reported using pirfenidone or nintedanib on enrollment into the trial were in the primary analysis. Spirometry was scheduled at baseline and the 12- and 24-month study visits. Linear mixed-effects models with random intercept and slope were used to examine changes in FVC over time. Models were adjusted for age, sex, smoking history, coronary artery disease history, baseline FVC, and 12-month spline term. Survival and nonelective respiratory hospitalization by antifibrotic type were determined using Cox regression models with adjustment for age, sex, smoking history, coronary artery disease history, and baseline FVC and diffusing capacity for carbon monoxide.
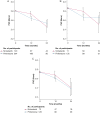
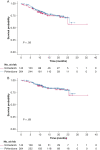
Results: Out of the 513 participants with IPF randomized in the CleanUP-IPF trial, 407 reported using pirfenidone (n = 264, 65%) or nintedanib (n = 143, 35%). The pirfenidone group had more participants with a history of coronary artery disease than the nintedanib group (34.1% vs 20.3%, respectively). Patients treated with nintedanib had a higher 12-month visit FVC than patients treated with pirfenidone (mean difference, 106 mL; 95% CI, 34-178). This difference was attenuated at the 24-month study visit. There were no significant differences in overall survival and nonelective respiratory hospitalization between the pirfenidone- and nintedanib-treated groups.
Interpretation: Patients with IPF who used nintedanib had a slower 12-month FVC decline than pirfenidone in a post hoc analysis of a clinical trial.
Keywords: antifibrotic; lung function; pulmonary fibrosis.
Copyright © 2023 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial/Nonfinancial Disclosures The authors have reported to CHEST the following: K. R. F. reports receiving personal fees from Boehringer Ingelheim, Roche/Genentech, Bellerophon, Shionogi, DevPro, AstraZeneca, Pure Health, Horizon, FibroGen, Sun Pharmaceuticals, Pliant, United Therapeutics, Arrowhead, Lupin, Polarean, PureTech, Trevi Therapeutics, CSL Behring, Daewoong, DisperSol, Immumet, NeRRe Therapeutics, Insilco, and Vicore outside the submitted work. F. J. M. reports grant support from the NHLBI that supported the data collection of the parent study; grant or contracts from Afferent/Merck, Boehringer Ingelheim, Biogen, Bristol Myers Squibb, DevPro, Nitto, Patara/Respivant, and ProMedior/Roche; consult fees from Boehringer Ingelheim, Bristol Myers Squibb, Hoffman/Laroche, IQVIA, Lung Therapuetics, Novartis, Sanofi, Shionogi, Two XR, and Veracyte; payment or honoraria from Boehringer Ingelheim; support for attending meetings/travel from Boehringer Ingelehim for international meetings; and participation of data safety monitoring board for Boehringer Ingelheim and Biogen. I. N. reports personal fees from Boehringer Ingelheim, Genentech, and Confo, outside the submitted work; and has a patent transcriptomic prognostics in IPF pending. None declared (J. S. K., S. M., E. Y., K. J. A., H. J. K.).
Figures
References
-
- King T.E., Jr., Bradford W.Z., Castro-Bernardini S., et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. - PubMed
-
- Richeldi L., du Bois R.M., Raghu G., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. - PubMed
-
- Dempsey T.M., Sangaralingham L.R., Yao X., Sanghavi D., Shah N.D., Limper A.H. Clinical effectiveness of antifibrotic medications for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;200(2):168–174. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

