Illuminating the mechanism and allosteric behavior of NanoLuc luciferase
- PMID: 38030625
- PMCID: PMC10687086
- DOI: 10.1038/s41467-023-43403-y
Illuminating the mechanism and allosteric behavior of NanoLuc luciferase
Abstract
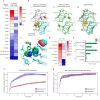
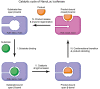
NanoLuc, a superior β-barrel fold luciferase, was engineered 10 years ago but the nature of its catalysis remains puzzling. Here experimental and computational techniques are combined, revealing that imidazopyrazinone luciferins bind to an intra-barrel catalytic site but also to an allosteric site shaped on the enzyme surface. Structurally, binding to the allosteric site prevents simultaneous binding to the catalytic site, and vice versa, through concerted conformational changes. We demonstrate that restructuration of the allosteric site can boost the luminescent reaction in the remote active site. Mechanistically, an intra-barrel arginine coordinates the imidazopyrazinone component of luciferin, which reacts with O2 via a radical charge-transfer mechanism, and then it also protonates the resulting excited amide product to form a light-emitting neutral species. Concomitantly, an aspartate, supported by two tyrosines, fine-tunes the blue color emitter to secure a high emission intensity. This information is critical to engineering the next-generation of ultrasensitive bioluminescent reporters.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

