A click chemistry-mediated all-peptide cell printing hydrogel platform for diabetic wound healing
- PMID: 38030636
- PMCID: PMC10687272
- DOI: 10.1038/s41467-023-43364-2
A click chemistry-mediated all-peptide cell printing hydrogel platform for diabetic wound healing
Abstract
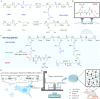
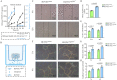
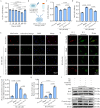
High glucose-induced vascular endothelial injury is a major pathological factor involved in non-healing diabetic wounds. To interrupt this pathological process, we design an all-peptide printable hydrogel platform based on highly efficient and precise one-step click chemistry of thiolated γ-polyglutamic acid, glycidyl methacrylate-conjugated γ-polyglutamic acid, and thiolated arginine-glycine-aspartate sequences. Vascular endothelial growth factor 165-overexpressed human umbilical vein endothelial cells are printed using this platform, hence fabricating a living material with high cell viability and precise cell spatial distribution control. This cell-laden hydrogel platform accelerates the diabetic wound healing of rats based on the unabated vascular endothelial growth factor 165 release, which promotes angiogenesis and alleviates damages on vascular endothelial mitochondria, thereby reducing tissue hypoxia, downregulating inflammation, and facilitating extracellular matrix remodeling. Together, this study offers a promising strategy for fabricating tissue-friendly, high-efficient, and accurate 3D printed all-peptide hydrogel platform for cell delivery and self-renewable growth factor therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Medical device: diabetic foot ulcer (DFU) treatment market. Available at: https://www.fortunebusinessinsights.com/industry-reports/diabetic-foot-u... (2020).
Publication types
MeSH terms
Substances
Grants and funding
- 82270595/National Natural Science Foundation of China (National Science Foundation of China)
- 82300648/National Natural Science Foundation of China (National Science Foundation of China)
- 32171402/National Natural Science Foundation of China (National Science Foundation of China)
- BX20220393, and 2022M723891/China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

