FOXP3 recognizes microsatellites and bridges DNA through multimerization
- PMID: 38030726
- PMCID: PMC10719092
- DOI: 10.1038/s41586-023-06793-z
FOXP3 recognizes microsatellites and bridges DNA through multimerization
Abstract
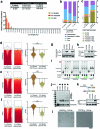
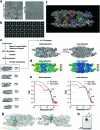
FOXP3 is a transcription factor that is essential for the development of regulatory T cells, a branch of T cells that suppress excessive inflammation and autoimmunity1-5. However, the molecular mechanisms of FOXP3 remain unclear. Here we here show that FOXP3 uses the forkhead domain-a DNA-binding domain that is commonly thought to function as a monomer or dimer-to form a higher-order multimer after binding to TnG repeat microsatellites. The cryo-electron microscopy structure of FOXP3 in a complex with T3G repeats reveals a ladder-like architecture, whereby two double-stranded DNA molecules form the two 'side rails' bridged by five pairs of FOXP3 molecules, with each pair forming a 'rung'. Each FOXP3 subunit occupies TGTTTGT within the repeats in a manner that is indistinguishable from that of FOXP3 bound to the forkhead consensus motif (TGTTTAC). Mutations in the intra-rung interface impair TnG repeat recognition, DNA bridging and the cellular functions of FOXP3, all without affecting binding to the forkhead consensus motif. FOXP3 can tolerate variable inter-rung spacings, explaining its broad specificity for TnG-repeat-like sequences in vivo and in vitro. Both FOXP3 orthologues and paralogues show similar TnG repeat recognition and DNA bridging. These findings therefore reveal a mode of DNA recognition that involves transcription factor homomultimerization and DNA bridging, and further implicates microsatellites in transcriptional regulation and diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









Update of
-
FoxP3 recognizes microsatellites and bridges DNA through multimerization.bioRxiv [Preprint]. 2023 Nov 10:2023.07.12.548762. doi: 10.1101/2023.07.12.548762. bioRxiv. 2023. Update in: Nature. 2023 Dec;624(7991):433-441. doi: 10.1038/s41586-023-06793-z. PMID: 37986949 Free PMC article. Updated. Preprint.
Comment in
-
An immune-cell transcription factor tethers DNA together.Nature. 2023 Dec;624(7991):255-256. doi: 10.1038/d41586-023-03628-9. Nature. 2023. PMID: 38030764 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

