Beet red food colourant can be produced more sustainably with engineered Yarrowia lipolytica
- PMID: 38030899
- PMCID: PMC10686825
- DOI: 10.1038/s41564-023-01517-5
Beet red food colourant can be produced more sustainably with engineered Yarrowia lipolytica
Abstract
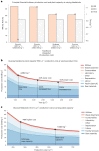
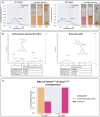
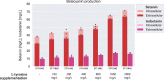
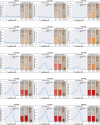
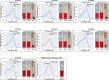
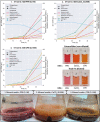
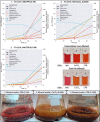
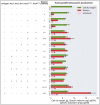
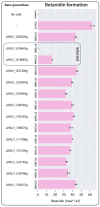
Synthetic food colourants are widely used in the food industry, but consumer concerns about safety and sustainability are driving a need for natural food-colour alternatives. Betanin, which is extracted from red beetroots, is a commonly used natural red food colour. However, the betanin content of beetroot is very low (~0.2% wet weight), which means that the extraction of betanin is incredibly wasteful in terms of land use, processing costs and vegetable waste. Here we developed a sustainability-driven biotechnological process for producing red beet betalains, namely, betanin and its isomer isobetanin, by engineering the oleaginous yeast Yarrowia lipolytica. Metabolic engineering and fermentation optimization enabled production of 1,271 ± 141 mg l-1 betanin and 55 ± 7 mg l-1 isobetanin in 51 h using glucose as carbon source in controlled fed-batch fermentations. According to a life cycle assessment, at industrial scale (550 t yr-1), our fermentation process would require significantly less land, energy and resources compared with the traditional extraction of betanin from beetroot crops. Finally, we apply techno-economic assessment to show that betanin production by fermentation could be economically feasible in the existing market conditions.
© 2023. The Author(s).
Conflict of interest statement
I.B., M.B., P.T.T. and M.C.P. are co-inventors on a patent application related to this study. The other authors declare no competing interests.
Figures







References
-
- Natural Food Colors Market Size Global Report, 2022–2030 (Polaris Market Research, 2022); https://www.polarismarketresearch.com/industry-analysis/natural-food-col...
-
- European Food Safety Authority (EFSA) Scientific opinion on the re-evaluation of beetroot red (E 162) as a food additive. EFSA Journal13, 4318 (2015).
-
- Gebhardt B, Sperl R, Carle R, Müller-Maatsch J. Assessing the sustainability of natural and artificial food colorants. J. Clean. Prod. 2020;260:120884. doi: 10.1016/j.jclepro.2020.120884. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- NNF21OC0072559/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20CC0035580/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0060809/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF21OC0072559/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20CC0035580/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0060809/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF21OC0072559/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20CC0035580/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0060809/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF21OC0072559/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20CC0035580/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0060809/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF21OC0072559/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20CC0035580/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0060809/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF21OC0072559/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20CC0035580/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0060809/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF21OC0072559/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20CC0035580/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0060809/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF21OC0072559/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20CC0035580/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0060809/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF21OC0072559/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20CC0035580/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0060809/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF21OC0072559/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20CC0035580/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0060809/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF21OC0072559/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20CC0035580/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0060809/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF21OC0072559/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20CC0035580/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0060809/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF21OC0072559/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20CC0035580/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0060809/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF21OC0072559/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20CC0035580/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF20OC0060809/Novo Nordisk Fonden (Novo Nordisk Foundation)
LinkOut - more resources
Full Text Sources

