Postmarket safety profile of suicide/self-injury for GLP-1 receptor agonist: a real-world pharmacovigilance analysis
- PMID: 38031404
- PMCID: PMC10755578
- DOI: 10.1192/j.eurpsy.2023.2474
Postmarket safety profile of suicide/self-injury for GLP-1 receptor agonist: a real-world pharmacovigilance analysis
Abstract
Background: Recent reports of individuals experiencing suicidal and/or self-injurious behaviors while using liraglutide and semaglutide have heightened the concerns regarding neuropsychiatric safety of Glucagon-like peptide-1 agonists (GLP-1RAs). As real-world evidence is very limited, we explored the association between GLP-1RA and suicide/self-injury by mining the FDA Adverse Event Reporting System (FAERS) database.
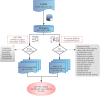
Methods: The FAERS database was queried from 2005 Q2 to 2023 Q2. The Reporting Odds Ratio (ROR) and Empirical Bayes Geometric Mean (EBGM) were used to conduct the disproportionality analysis.
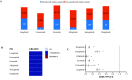
Results: A total of 534 GLP-1RA-associated suicide/self-injury cases were reported in the FAERS during the study period. GLP-1RA did not cause a disproportionate increase in overall suicidal and self-injurious cases (ROR: 0.16, 95%CI 0.15-0.18, P < 0.001; EBGM05: 0.15). Stratified analyses found no safety signal of suicide/injury for GLP-1RA in both females and males. The ROR for suicide/self-injury with GLP-1RA was slightly elevated (ROR: 2.50, 95%CI 1.02-6.13, P = 0.05) in children, while the EBGM05 was < 2 in this population. No significant signal value was observed in other age groups. No over-reporting of suicide/self-injury was identified for GLP-1RA before or after the COVID-19 pandemic outbreak.
Conclusions: The cases of suicide or self-injury reported to FAERS do not indicate any overall safety signal attributable to GLP-1RA at this time. Subgroup analysis revealed a marginal elevation of ROR for suicide and self-injury with GLP-1RA in children, but no safety signal was detected by EBGM05 in this population. Further large-scale prospective investigations are still warranted to further confirm this finding.
Keywords: FAERS; GLP-1RA; pharmacovigilance analysis; self-injury; suicide.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Mortality and Serious Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists: A Pharmacovigilance Study Using the FDA Adverse Event Reporting System.Cureus. 2024 Aug 2;16(8):e65989. doi: 10.7759/cureus.65989. eCollection 2024 Aug. Cureus. 2024. PMID: 39221363 Free PMC article.
-
Neuropsychiatric adverse events associated with Glucagon-like peptide-1 receptor agonists: a pharmacovigilance analysis of the FDA Adverse Event Reporting System database.Eur Psychiatry. 2025 Feb 4;68(1):e20. doi: 10.1192/j.eurpsy.2024.1803. Eur Psychiatry. 2025. PMID: 39901452 Free PMC article.
-
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and suicidality: A replication study using reports to the World Health Organization pharmacovigilance database (VigiBase®).J Affect Disord. 2025 Jan 15;369:922-927. doi: 10.1016/j.jad.2024.10.062. Epub 2024 Oct 19. J Affect Disord. 2025. PMID: 39433133
-
Association of GLP-1 Receptor Agonists With Risk of Suicidal Ideation and Behaviour: A Systematic Review and Meta-Analysis.Diabetes Metab Res Rev. 2025 Feb;41(2):e70037. doi: 10.1002/dmrr.70037. Diabetes Metab Res Rev. 2025. PMID: 39945396 Free PMC article.
-
Risk factors of immune checkpoint inhibitor-associated acute kidney injury: evidence from clinical studies and FDA pharmacovigilance database.BMC Nephrol. 2023 Apr 22;24(1):107. doi: 10.1186/s12882-023-03171-9. BMC Nephrol. 2023. PMID: 37087434 Free PMC article.
Cited by
-
Exploration of the potential association between GLP-1 receptor agonists and suicidal or self-injurious behaviors: a pharmacovigilance study based on the FDA Adverse Event Reporting System database.BMC Med. 2024 Feb 14;22(1):65. doi: 10.1186/s12916-024-03274-6. BMC Med. 2024. PMID: 38355513 Free PMC article.
-
Suicide and Self-Harm Events With GLP-1 Receptor Agonists in Adults With Diabetes or Obesity: A Systematic Review and Meta-Analysis.JAMA Psychiatry. 2025 Mar 19:e250091. doi: 10.1001/jamapsychiatry.2025.0091. Online ahead of print. JAMA Psychiatry. 2025. PMID: 40105856
-
Suicide and suicide attempt in users of GLP-1 receptor agonists: a nationwide case-time-control study.EClinicalMedicine. 2024 Dec 31;80:103029. doi: 10.1016/j.eclinm.2024.103029. eCollection 2025 Feb. EClinicalMedicine. 2024. PMID: 39844933 Free PMC article.
-
Antidepressants and Weight Gain: An Update on the Evidence and Clinical Implications.Curr Obes Rep. 2025 Jan 3;14(1):2. doi: 10.1007/s13679-024-00598-5. Curr Obes Rep. 2025. PMID: 39753939 Review.
-
Association of Vancomycin Plus Piperacillin-Tazobactam With Acute Kidney Injury: Differentiating Pseudo-Injury From True Nephrotoxicity.Clin Transl Sci. 2025 May;18(5):e70258. doi: 10.1111/cts.70258. Clin Transl Sci. 2025. PMID: 40391975 Free PMC article.
References
-
- Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740–56. - PubMed
-
- Jepsen MM, Christensen MB. Emerging glucagon-like peptide 1 receptor agonists for the treatment of obesity. Expert Opin Emerg Drugs. 2021;26(3):231–43. - PubMed
-
- Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136(9):849–70. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

