The Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial: Protocol for school-age follow-up
- PMID: 38031545
- PMCID: PMC10685067
- DOI: 10.12688/wellcomeopenres.19463.1
The Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial: Protocol for school-age follow-up
Abstract
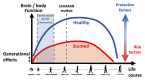
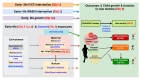
Background: There is a need for follow-up of early-life stunting intervention trials into childhood to determine their long-term impact. A holistic school-age assessment of health, growth, physical and cognitive function will help to comprehensively characterise the sustained effects of early-life interventions. Methods: The Sanitation Hygiene Infant Nutrition Efficacy (SHINE) trial in rural Zimbabwe assessed the effects of improved infant and young child feeding (IYCF) and/or improved water, sanitation and hygiene (WASH) on stunting and anaemia at 18 months. Among children enrolled to SHINE, 1,275 have been followed up at 7-8 years of age (1,000 children who have not been exposed to HIV, 268 exposed to HIV antenatally who remain HIV negative and 7 HIV positive children). Children were assessed using the School-Age Health, Activity, Resilience, Anthropometry and Neurocognitive (SAHARAN) toolbox, to measure their growth, body composition, cognitive and physical function. In parallel, a caregiver questionnaire assessed household demographics, socioeconomic status, adversity, nurturing, caregiver support, food and water insecurity. A monthly morbidity questionnaire is currently being administered by community health workers to evaluate school-age rates of infection and healthcare-seeking. The impact of the SHINE IYCF and WASH interventions, the early-life 'exposome', maternal HIV, and contemporary exposures on each school-age outcome will be assessed. We will also undertake an exploratory factor analysis to generate new, simpler metrics for assessment of cognition (COG-SAHARAN), growth (GROW-SAHARAN) and combined growth, cognitive and physical function (SUB-SAHARAN). The SUB-SAHARAN toolbox will be used to conduct annual assessments within the SHINE cohort from ages 8-12 years. Ethics and dissemination: Approval was obtained from Medical Research Council of Zimbabwe (08/02/21) and registered with Pan-African Clinical Trials Registry (PACTR202201828512110, 24/01/22). Primary caregivers provided written informed consent and children written assent. Findings will be disseminated through community sensitisation, peer-reviewed journals and stakeholders including the Zimbabwean Ministry of Health and Child Care.
Keywords: Child; HIV; IYCF; WASH; body composition; cognition; fitness; nutrition.
Copyright: © 2023 Piper JD et al.
Conflict of interest statement
No competing interests were disclosed.
Figures

References
-
- UNICEF, WHO, World Bank Group: Levels and Trends in Child Malnutrition. In: edition JCME, ed.,2017. Reference Source
-
- Das JK, Salam RA, Hadi YB, et al. : Preventive lipid-based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes. Cochrane Database Syst Rev. 2019;5(5): CD012611. 10.1002/14651858.CD012611.pub3 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

