Adverse effects of immunotherapies for multiple sclerosis: a network meta-analysis
- PMID: 38032059
- PMCID: PMC10687854
- DOI: 10.1002/14651858.CD012186.pub2
Adverse effects of immunotherapies for multiple sclerosis: a network meta-analysis
Abstract
Background: Multiple sclerosis (MS) is a chronic disease of the central nervous system that affects mainly young adults (two to three times more frequently in women than in men) and causes significant disability after onset. Although it is accepted that immunotherapies for people with MS decrease disease activity, uncertainty regarding their relative safety remains.
Objectives: To compare adverse effects of immunotherapies for people with MS or clinically isolated syndrome (CIS), and to rank these treatments according to their relative risks of adverse effects through network meta-analyses (NMAs).
Search methods: We searched CENTRAL, PubMed, Embase, two other databases and trials registers up to March 2022, together with reference checking and citation searching to identify additional studies.
Selection criteria: We included participants 18 years of age or older with a diagnosis of MS or CIS, according to any accepted diagnostic criteria, who were included in randomized controlled trials (RCTs) that examined one or more of the agents used in MS or CIS, and compared them versus placebo or another active agent. We excluded RCTs in which a drug regimen was compared with a different regimen of the same drug without another active agent or placebo as a control arm.
Data collection and analysis: We used standard Cochrane methods for data extraction and pairwise meta-analyses. For NMAs, we used the netmeta suite of commands in R to fit random-effects NMAs assuming a common between-study variance. We used the CINeMA platform to GRADE the certainty of the body of evidence in NMAs. We considered a relative risk (RR) of 1.5 as a non-inferiority safety threshold compared to placebo. We assessed the certainty of evidence for primary outcomes within the NMA according to GRADE, as very low, low, moderate or high.
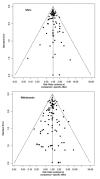
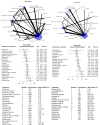
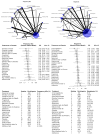
Main results: This NMA included 123 trials with 57,682 participants. Serious adverse events (SAEs) Reporting of SAEs was available from 84 studies including 5696 (11%) events in 51,833 (89.9%) participants out of 57,682 participants in all studies. Based on the absolute frequency of SAEs, our non-inferiority threshold (up to a 50% increased risk) meant that no more than 1 in 18 additional people would have a SAE compared to placebo. Low-certainty evidence suggested that three drugs may decrease SAEs compared to placebo (relative risk [RR], 95% confidence interval [CI]): interferon beta-1a (Avonex) (0.78, 0.66 to 0.94); dimethyl fumarate (0.79, 0.67 to 0.93), and glatiramer acetate (0.84, 0.72 to 0.98). Several drugs met our non-inferiority criterion versus placebo: moderate-certainty evidence for teriflunomide (1.08, 0.88 to 1.31); low-certainty evidence for ocrelizumab (0.85, 0.67 to 1.07), ozanimod (0.88, 0.59 to 1.33), interferon beta-1b (0.94, 0.78 to 1.12), interferon beta-1a (Rebif) (0.96, 0.80 to 1.15), natalizumab (0.97, 0.79 to 1.19), fingolimod (1.05, 0.92 to 1.20) and laquinimod (1.06, 0.83 to 1.34); very low-certainty evidence for daclizumab (0.83, 0.68 to 1.02). Non-inferiority with placebo was not met due to imprecision for the other drugs: low-certainty evidence for cladribine (1.10, 0.79 to 1.52), siponimod (1.20, 0.95 to 1.51), ofatumumab (1.26, 0.88 to 1.79) and rituximab (1.01, 0.67 to 1.52); very low-certainty evidence for immunoglobulins (1.05, 0.33 to 3.32), diroximel fumarate (1.05, 0.23 to 4.69), peg-interferon beta-1a (1.07, 0.66 to 1.74), alemtuzumab (1.16, 0.85 to 1.60), interferons (1.62, 0.21 to 12.72) and azathioprine (3.62, 0.76 to 17.19). Withdrawals due to adverse events Reporting of withdrawals due to AEs was available from 105 studies (85.4%) including 3537 (6.39%) events in 55,320 (95.9%) patients out of 57,682 patients in all studies. Based on the absolute frequency of withdrawals, our non-inferiority threshold (up to a 50% increased risk) meant that no more than 1 in 31 additional people would withdraw compared to placebo. No drug reduced withdrawals due to adverse events when compared with placebo. There was very low-certainty evidence (meaning that estimates are not reliable) that two drugs met our non-inferiority criterion versus placebo, assuming an upper 95% CI RR limit of 1.5: diroximel fumarate (0.38, 0.11 to 1.27) and alemtuzumab (0.63, 0.33 to 1.19). Non-inferiority with placebo was not met due to imprecision for the following drugs: low-certainty evidence for ofatumumab (1.50, 0.87 to 2.59); very low-certainty evidence for methotrexate (0.94, 0.02 to 46.70), corticosteroids (1.05, 0.16 to 7.14), ozanimod (1.06, 0.58 to 1.93), natalizumab (1.20, 0.77 to 1.85), ocrelizumab (1.32, 0.81 to 2.14), dimethyl fumarate (1.34, 0.96 to 1.86), siponimod (1.63, 0.96 to 2.79), rituximab (1.63, 0.53 to 5.00), cladribine (1.80, 0.89 to 3.62), mitoxantrone (2.11, 0.50 to 8.87), interferons (3.47, 0.95 to 12.72), and cyclophosphamide (3.86, 0.45 to 33.50). Eleven drugs may have increased withdrawals due to adverse events compared with placebo: low-certainty evidence for teriflunomide (1.37, 1.01 to 1.85), glatiramer acetate (1.76, 1.36 to 2.26), fingolimod (1.79, 1.40 to 2.28), interferon beta-1a (Rebif) (2.15, 1.58 to 2.93), daclizumab (2.19, 1.31 to 3.65) and interferon beta-1b (2.59, 1.87 to 3.77); very low-certainty evidence for laquinimod (1.42, 1.01 to 2.00), interferon beta-1a (Avonex) (1.54, 1.13 to 2.10), immunoglobulins (1.87, 1.01 to 3.45), peg-interferon beta-1a (3.46, 1.44 to 8.33) and azathioprine (6.95, 2.57 to 18.78); however, very low-certainty evidence is unreliable. Sensitivity analyses including only studies with low attrition bias, drug dose above the group median, or only patients with relapsing remitting MS or CIS, and subgroup analyses by prior disease-modifying treatments did not change these figures. Rankings No drug yielded consistent P scores in the upper quartile of the probability of being better than others for primary and secondary outcomes.
Authors' conclusions: We found mostly low and very low-certainty evidence that drugs used to treat MS may not increase SAEs, but may increase withdrawals compared with placebo. The results suggest that there is no important difference in the occurrence of SAEs between first- and second-line drugs and between oral, injectable, or infused drugs, compared with placebo. Our review, along with other work in the literature, confirms poor-quality reporting of adverse events from RCTs of interventions. At the least, future studies should follow the CONSORT recommendations about reporting harm-related issues. To address adverse effects, future systematic reviews should also include non-randomized studies.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
IT ‐ none known.
GV ‐ no relevant interests; published research on this topic; works as a health professional at University of Florence and AOU Careggi, Italy; since 1 Jan 2021, Queen's University Belfast; local PI of the RHINE trial on faricimab for DMO on behalf of the University of Florence and Florence Careggi Hospital (did not sign the contract and funding was not under direct control; Roche funded the multicentre trial and Florence was a trial site).
VP ‐ none known.
EL ‐ none known.
MDB ‐ none known.
MC ‐ none known.
GC ‐ none known.
SF ‐ none known.
MGL ‐ none known.
RF ‐ financial assistance with personal prescription costs from Biogen Inc. (2018 to 2022); former member of Cochrane Central Editorial Service.
GF ‐ no relevant interests; Co‐ordinating Editor of Cochrane Multiple Sclerosis and Rare Diseases of the CNS but was excluded from the editorial and decision‐making process of this review.
Figures
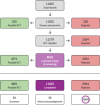
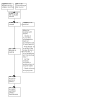
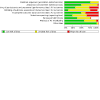

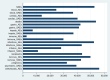















Update of
- doi: 10.1002/14651858.CD012186
References
References to studies included in this review
Achiron 1998 {published data only}
-
- Achiron A, Gabbay U, Gilad R, Hassin B, Barak Y, Gornish M, et al. Intravenous immunoglobulin treatment in multiple sclerosis. Effect on relapses. Neurology 1998;50(2):398-402. - PubMed
Achiron 2004 {published data only}
-
- Achiron A, Kishner I, Sarova-Pinhas I, Raz H, Faibel M, Stern Y, et al. Intravenous immunoglobulin treatment following the first demyelinating event suggestive of multiplesclerosis: a randomized, double-blind, placebo-controlled trial. Archives of Neurology 2004;61(10):1515-20. - PubMed
ADVANCE 2014 {published data only}
-
- Calabresi PA, Kieseier BC, Arnold DL, Balcer LJ, Boyko A, Pelletier J, et al. Pegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. The Lancet Neurology 2014;13:657-65. Supplementary webappendix; p. 1-16. - PubMed
-
- NCT00906399. Efficacy and safety study of Peginterferon beta-1a in participants with relapsing multiple sclerosis (ADVANCE). clinicaltrials.gov/show/NCT00906399 (first posted May 21, 2009).
-
- Newsome SD, Guo S, Altincatal A, Proskorovsky I, Kinter E, Phillips G, et al. Impact of peginterferon beta-1a and disease factors on quality of life in multiple sclerosis. Multiple Sclerosis and Related Disorders 2015;4(4):350-7. - PubMed
AFFIRM 2006 {published data only}
-
- Lublin FD, Cutter G, Giovannoni G, Pace A, Campbell NR, Belachew S. Natalizumab reduces relapse clinical severity and improves relapse recovery in MS. Multiple Sclerosis and Related Disorders 2014;3(6):705-11. - PubMed
-
- NCT00027300. Safety and efficacy of natalizumab in the treatment of multiple sclerosis [A randomized, double-blind, placebo-controlled, parallel-group, multicenter study to determine the safety and efficacy of natalizumab in subjects with relapsing-remitting multiple sclerosis]. clinicaltrials.gov/show/NCT00027300 (first received 3 December 2001).
-
- Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. New England Journal of Medicine 2006;354(9):899-910. - PubMed
ALLEGRO 2012 {published data only}
-
- Comi G, Jeffery D, Kappos L, Montalban X, Boyko A, Rocca MA, et al. Placebo-controlled trial of oral laquinimod for multiple sclerosis. New England Journal of Medicine 2012;366(11):1000-9. Supplementary Appendix; Trial protocol; p. 1-352. - PubMed
-
- NCT00509145. Safety and efficacy of orally administered laquinimod versus placebo for treatment of relapsing remitting multiple sclerosis (RRMS) (ALLEGRO) [A multinational, multicenter, randomized, double-blind, parallel-group, placebo-controlled study, to evaluate the safety, tolerability and efficacy of daily oral administration of Laquinimod 0.6 mg in subjects with RRMS]. clinicaltrials.gov/show/NCT00509145 (first received 31 July 2007).
Andersen 2004 {published data only}
-
- Andersen O, Elovaara I, Farkkila M, Hansen HJ, Mellgren SI, Myhr KM, et al. Multicentre, randomised, double blind, placebo controlled, phase III study of weekly, low dose, subcutaneous interferon beta-1a in secondary progressive multiple sclerosis. Journal of Neurology, Neurosurgery and Psychiatry 2004;75(5):706-10. - PMC - PubMed
APEX 2019 {published data only}
-
- Kondo T, Kawachi I, Onizuka Y, Hiramatsu K, Hase M, Yun J, et al. Efficacy of dimethyl fumarate in Japanese multiple sclerosis patients: interim analysis of randomized, double-blind APEX study and its open-label extension. Multiple Sclerosis Journal – Experimental, Translational and Clinical 2019;5(3):2055217319864974. - PMC - PubMed
-
- Mori M, Ohashi T, Onizuka Y, Hiramatsu K, Hase M, Yun J, et al. Efficacy and safety of delayed-release dimethyl fumarate in treatment-naïve Japanese patients with relapsing-remitting multiple sclerosis: A post-hoc subgroup analysis of the APEX study. Journal of the Neurological Sciences 2017;381:795-6.
-
- Mori M, Ohashi T, Onizuka Y, Hiramatsu K, Hase M, Yun J, et al. Efficacy and safety of dimethyl fumarate in Japanese MS patients who had the history of treatment with fingolimod: APEX part 1+2 interim analysis. Multiple Sclerosis 2019;25(3):457-8.
-
- Mori M, Ohashi T, Onizuka Y, Hiramatsu K, Hase M, Yun J, et al. Efficacy and safety of dimethyl fumarate in treatment-naïve Japanese patients with multiple sclerosis: Interim analysis of the randomized placebo-controlled study. Multiple Sclerosis Journal – Experimental, Translational and Clinical 2019;5(2):2055217319852727. - PMC - PubMed
-
- NCT01838668. An efficacy and safety study of BG00012 (dimethyl fumarate) in Asian subjects with relapsing remitting multiple sclerosis (RRMS) [A multicenter, randomized, double-blind, placebo-controlled, efficacy and safety study of BG00012 in subjects from the Asia-Pacific region and other countries with relapsing-remitting multiple sclerosis]. clinicaltrials.gov/show/NCT01838668 (first received 24 April 2013).
APOLITOS 2021 {published data only}
-
- Kira JI, Nakahara J, Sazonov DV, Kurosawa T, Tsumiyama I, Willi R, et al. Effect of ofatumumab versus placebo in relapsing multiple sclerosis patients from Japan and Russia: Phase 2 APOLITOS study. Multiple sclerosis (Houndmills, Basingstoke, England) 2022;28(8):1229-38. Supplemental material [Figure e-1; Table e-1]. - PubMed
-
- NCT03249714. Efficacy and safety of ofatumumab compared to placebo in patients with relapsing multiple sclerosis followed by extended treatment with open-label ofatumumab [A 24-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter study to evaluate the efficacy, safety and pharmacokinetics of Ofatumumab in patients with relapsing multiple sclerosis followed by an extended treatment of at least 24 weeks with open-label Ofatumumab i]. clinicaltrials.gov/show/NCT03249714 (first received 15 August 2017).
ARPEGGIO 2020 {published data only}
-
- Giovannoni G, Barkhof F, Hartung HP, Cree B, Krieger S, Montalban X, et al. Arpeggio: a placebo-controlled trial of oral laquinimod in primary progressive multiple sclerosis. Neurology 2018;90(15 Supplement):S8.003. - PubMed
-
- Giovannoni G, Knappertz V, Steinerman JR, Tansy AP, Li T, Krieger S, et al. A randomized, placebo-controlled, phase 2 trial of laquinimod in primary progressive multiple sclerosis. Neurology 2020;95(8):e1027-40. Figure e-2. - PubMed
-
- NCT02284568. A phase 2 clinical study in subjects with primary progressive multiple sclerosis to assess the efficacy, safety and tolerability of two oral doses of laquinimod either of 0.6 mg/day or 1.5mg/day (experimental drug) as compared to placebo (ARPEGGIO) [A 24-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter study to evaluate the efficacy, safety and pharmacokinetics of Ofatumumab in patients with relapsing multiple sclerosis followed by an extended treatment of at least 24 weeks with open-label Ofatumumab]. clinicaltrials.gov/show/NCT02284568 (first received 6 November 2014).
ASCEND 2018 {published data only}
-
- Cano S, Cleanthous S, Marquis P, Petrillo J, Steiner D, Watson C, et al. Measuring the impact of secondary progressive multiple sclerosis (Spms) in the Ascend trial: equating the Msis-29, Msws-12, Abilhand-56 and Sf-36. Value Health 2015;18(7):A713.
-
- Kapoor R, Ho PR, Campbell N, Chang I, Deykin A, Forrestal F, et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurology 2018;17(5):405-15. Appendix, p. 1-14. - PubMed
-
- NCT01416181. A clinical study of the efficacy of natalizumab on reducing disability progression in participants with secondary progressive multiple sclerosis (ASCEND in SPMS) [A multicenter, randomized, double-blind, placebo-controlled study of the efficacy of natalizumab on reducing disability progression in subjects with secondary progressive multiple sclerosis, with optional open-label extension]. clinicaltrials.gov/show/NCT01416181 (first received 12 August 2011).
ASCLEPIOS I 2020 {published data only}
-
- Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, et al. Ofatumumab versus teriflunomide in multiple sclerosis. New England Journal of Medicine 2020;383(6):546-57. Appendix; Figure S4. Protocol; p. 1-61. - PubMed
-
- NCT02792218. Efficacy and safety of Ofatumumab compared to Teriflunomide in patients with relapsing multiple sclerosis (ASCLEPIOS I) [A randomized, double-blind, double-dummy, parallel-group study comparing the efficacy and safety of Ofatumumab versus Teriflunomide in patients with relapsing multiple sclerosis]. clinicaltrials.gov/show/NCT02792218 (first received 7 June 2016).
ASCLEPIOS II 2020 {published data only}
-
- Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, et al. Ofatumumab versus teriflunomide in multiple sclerosis. New England Journal of Medicine 2020;383(6):546-57. Figure S4. - PubMed
-
- NCT02792231. Efficacy and safety of ofatumumab compared to teriflunomide in patients with relapsing multiple sclerosis. (ASCLEPIOS II) [A randomized, double-blind, double-dummy, parallel-group study comparing the efficacy and safety of Ofatumumab versus Teriflunomide in patients with relapsing multiple sclerosis]. clinicaltrials.gov/show/NCT02792231 (first received 7 June 2016).
Ashtari 2011 {published data only}
ASSESS 2020 {published data only}
-
- Cree BAC, Goldman MD, Corboy JR, Singer BA, Fox EJ, Arnold DL, et al. Efficacy and safety of 2 fingolimod doses vs glatiramer acetate for the treatment of patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA Neurology 2020;78(1):1-13. Appendix, [Protocol]; p.1-96. - PMC - PubMed
-
- NCT01633112. MS study evaluating safety and efficacy of two doses of fingolimod versus copaxone (ASSESS) [A 12-month, randomized, rater- and dose-blinded study to compare the efficacy and safety of Fingolimod 0.25 mg and 0.5 mg administered orally once daily with Glatiramer acetate 20 mg administered subcutaneously once daily in patients with relapsing-remitting multiple sclerosis]. clinicaltrials.gov/show/NCT01633112 (first received 4 July 2012).
AVANTAGE 2013 {published data only}
-
- International Conference on Harmonisation (ICH GCP). The AVANTAGE study - A randomized, multicenter, phase iv, open-label prospective study comparing injection site reaction and injection site pain in patients with relapsing remitting multiple sclerosis (RRMS) or after a first demyelinating event suggestive of MS newly started on interferon beta-1b (Betaferon®) or interferon beta-1a (Rebif®). https://ichgcp.net/clinical-trials-registry/NCT00317941 (accessed 6 June 2022) 2013.
-
- NCT00317941. Safety study in relapsing-remitting multiple sclerosis (RRMS) patients receiving Betaferon or Rebif [The AVANTAGE study - A randomized, multicenter, phase iv, open-label prospective study comparing injection site reaction and injection site pain in patients with relapsing remitting multiple sclerosis (RRMS) or after a first demyelinating event suggestive of MS newly started on interferon beta-1b (Betaferon®) or interferon beta-1a (Rebif®)]. ClinicalTrials.gov: NCT00317941 (first received: 25 April 2006).
BECOME 2009 {published data only}
-
- Cadavid D, Wolansky LJ, Skurnick J, Lincoln J, Cheriyan J, Szczepanowski K, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology 2009;72(23):1976-83. - PubMed
-
- NCT00176592. Phase IV study, betaseron versus copaxone for relapsing remitting or CIS forms of MS using triple dose Gad 3 T MRI (BECOME) [Phase IV, rater-blinded, randomized study, comparing 250 mg of Betaseron with 20 mg of Copaxone in patients with the relapsing-remitting(RR) or CIS forms of MS using 3 tesla (3t) magnetic resonance imaging (MRI) with triple-dose gadolinium]. clinicaltrials.gov/show/NCT00176592 (first received 15 September 2005).
BENEFIT 2006 {published data only}
-
- Kappos L, Polman CH, Freedman MS, Edan G, Hartung HP, Miller DH, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006;67(7):1242-9. - PubMed
BEYOND 2009 {published data only}
-
- NCT00099502. BEYOND: Betaferon/Betaseron efficacy yielding outcomes of a new dose in multiple sclerosis (MS) patients [International, randomized, multicenter, phase IIIB study in patients with relapsing-remitting multiple sclerosis comparing over a treatment period of at least 104 weeks: 1. double-blinded safety, tolerability, and efficacy of Betaseron/Betaferon 250 µg (8 miu) and Betaseron/Betaferon 500 µg (16 MIU), both given subcutaneously every other day, and 2. rater-blinded safety, tolerability, and efficacy of Betaseron/Betaferon s.c. every other day with Copaxone 20 mg s.c. once daily]. clinicaltrials.gov/show/NCT00099502 (first received 16 December 2004).
-
- O'Connor P, Filippi M, Arnason B, Comi G, Cook S, Goodin D, et al. 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurology 2009;8(10):889-97. - PubMed
BOLD 2013 {published data only}
-
- NCT00879658. Safety, tolerability, efficacy and optimal dose finding study of BAF312 in patients with relapsing-remitting multiple sclerosis [A phase II, double-blind, randomized, multi-center, adaptive dose-ranging, placebo-controlled, parallel-group study evaluating safety, tolerability and efficacy on MRI lesion parameters and determining the dose response curve of BAF312 given orally once daily in patients with relapsing-remitting multiple sclerosis]. ClinicalTrials.gov: NCT00879658 (first received 10 April 2009).
-
- Selmaj K, Li DK, Hartung HP, Hemmer B, Kappos L, Freedman MS, et al. Siponimod for patients with relapsing-remitting multiple sclerosis (BOLD): an adaptive, dose-ranging, randomised, phase 2 study. The Lancet. Neurology 2013;12(8):756-67. - PubMed
Bornstein 1987 {published data only}
-
- Bornstein MB, Miller A, Slagle S, Weitzman M, Crystal, Drexler E, et al. A pilot trial of Cop 1 in exacerbating-remitting multiple sclerosis. New England Journal of Medicine 1987;317(7):408-14. - PubMed
Bornstein 1991 {published data only}
-
- Bornstein MB, Miller A, Slagle S, Weitzman M, Drexler E, Keilson M. A placebo-controlled, double-blind, randomised, two-center, pilot trial of Cop 1 in chronic progressive multiple sclerosis. Neurology 1991;41:533-9. - PubMed
Boyko 2016 {published and unpublished data}
-
- Boyko AN, Lashch NY, Sharanova SN, Zakharova MN, Trifonova OV, Simaniv TO, et al. Comparative, placebo-controlled clinical study of efficacy and safety of glatiramer acetate 20 mg in patients with relapsing-remitting multiple sclerosis: results of the first year of the study [Sravnitel'noe platsebo-kontroliruemoe klinicheskoe issledovanie effektivnosti i bezopasnosti preparatov glatiramera atsetata 20 mg u patsientov s remittiruyushchim rasseyannym sklerozom: rezul'taty pervogo goda nablyudeniya]. Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova 2016;116(10):61-7. - PubMed
-
- NCT02753088. Efficacy and safety of BCD-063 and Copaxone-Teva in patients with relapsing-remitting multiple sclerosis [International, multicentre, double-blind, placebo-controlled, comparative, randomized study to compare efficacy and safety of the generic drug BCD-063 (CJSC "BIOCAD", Russia) and Copaxone®-Teva ("Teva Pharmaceutical Industries Limited", Israel) in patients with relapsing-remitting multiple sclerosis]. ClinicalTrials.gov/show/NCT02753088 (first received 27 April 2016).
Boyko 2017 {published data only}
-
- Boyko AN, Bosenko LP, Vasilovskiy VV, Volkova LI, Zakharova MN, Kotov SV, et al. A comparative placebo-controlled clinical study on the efficacy and safety of interferon beta-1a for subcutaneous injections in patients with remitting multiple sclerosis: results of the first year of observations [Sravnitel'noe platsebo-kontroliruemoe klinicheskoe issledovanie éffektivnosti i bezopasnosti preparatov interferona beta-1a dlia podkozhnogo vvedeniia u patsientov s remittiruiushchim rasseiannym sklerozom: rezul'taty pervogo goda nabliudeniia]. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova 2017;117(2):107-13. - PubMed
BPSM 1995 {published data only}
BRAVO 2014 {published data only}
-
- NCT00605215. BRAVO study: Laquinimod double blind placebo controlled study in RRMS patients with a rater blinded reference arm of Interferon β-1a (Avonex®) (BRAVO) [A multinational, multicenter, randomized, parallel-group study performed in subjects with relapsing-remitting multiple sclerosis (RRMS) to assess the efficacy, safety and tolerability of Laquinimod over placebo in a double-blind design and of a reference arm of Interferon β-1a (Avonex®) in a rater-blinded design]. clinicaltrials.gov/show/NCT00605215 (first received 30 January 2008).
-
- Nakamura K, Vollmer TL, Gorfine T, Knappertz V, Arnold DL. Effect of laquinimod on gray matter and white matter atrophy in relapsing-remitting multiple sclerosis: Analysis of the BRAVO phase III trial. Multiple Sclerosis 2014;20(1):84-5.
-
- Vollmer TL, Sorensen PS, Selmaj K, Zipp F, Havrdova E, Cohen JA, et al. A randomized placebo-controlled phase III trial of oral laquinimod for multiple sclerosis. Journal of Neurology 2014;261(4):773-83. Appendix, p. 1-10. - PubMed
British and Dutch 1988 {published data only}
-
- The British and Dutch MSATG. Double-masked trial of azathioprine in multiple sclerosis. Lancet 1988;2(8604):179-83. - PubMed
Calabrese 2012 {published data only}
-
- Calabrese M, Bernardi V, Atzori M, Mattisi I, Favaretto A, Rinaldi F, et al. Effect of disease-modifying drugs on cortical lesions and atrophy in relapsing-remitting multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2012;18(4):418-24. - PubMed
CAMMS223 2008 {published data only}
-
- CAMMS223 TI, Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. New England Journal of Medicine 2008;359(17):1786-801. - PubMed
-
- Fox EJ, Wynn D, Coles AJ, Palmer J, Margolin DH. ALEMTUZUMAB improves neurological functional systems in treatment-naive relapsing-remitting multiple sclerosis patients. Journal of the Neurological Sciences 2016;363:188-94. - PubMed
-
- NCT00050778. A phase II study comparing low- and high-dose alemtuzumab and high-dose Rebif® in patients with early, active relapsing-remitting multiple sclerosis [A phase II, randomized, open-label, three-arm study comparing low- and high-dose Alemtuzumab and high-dose subcutaneous Interferon beta-1a (Rebif®) in patients with early, active relapsing-remitting multiple sclerosis]. clinicaltrials.gov/show/NCT00050778 (first received 23 December 2002).
CARE‐MS I 2012 {published data only}
-
- Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380(9856):1819-28. - PubMed
-
- Krieger S, Lubetzki C, Palmer J, Margolin DH. Alemtuzumab reduces disease activity in treatment naive patients with highly active relapsing-remitting multiple sclerosis. Multiple Sclerosis 2014;20(1):106-7.
-
- Montalban X, Arnold DL, Fisher E, Margolin DH, Palmer J. Improvement in MRI outcomes across subgroups with alemtuzumab versus interferon beta-1a in treatment naive relapsing-remitting multiple sclerosis. Multiple Sclerosis 2014;20(1):83-4.
-
- NCT00530348. Comparison of Alemtuzumab and Rebif® efficacy in multiple sclerosis, study one (CARE-MS I) [A phase 3 randomized, rater-blinded study comparing two annual cycles of intravenous Alemtuzumab to three-times weekly subcutaneous Interferon beta-1a (Rebif®) in treatment-naïve patients with relapsing-remitting multiple sclerosis]. clinicaltrials.gov/show/NCT00530348 (first received 17 September 2007).
CARE‐MS II 2012 {published data only}
-
- Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380(9856):1829-39. - PubMed
-
- NCT00548405. Comparison of alemtuzumab and Rebif® efficacy in multiple sclerosis, study two (CARE-MS II) [A phase 3, randomized, rater- and dose-blinded study comparing two annual cycles of intravenous low- and high-dose Alemtuzumab to three-times weekly subcutaneous Interferon beta 1a (Rebif®) in patients with relapsing remitting multiple sclerosis who have relapsed on therapy]. clinicaltrials.gov/show/NCT00548405 (firstreceived 24 October 2007).
-
- Steinman L, Wang H, Liu Y, Palmer J, Zhang Q. Defining clinical meaning of patient-reported outcomes with disability assessment in multiple sclerosis: An analysis of the CARE-MS II study. Multiple Sclerosis 2014;20(1):419.
CCMSSG 1991 {published data only}
-
- CCMSSG. The Canadian cooperative trial of cyclophosphamide and plasma exchange in progressive multiple sclerosis. Lancet 1991;337(8739):441-6. - PubMed
CHAMPS 2000 {published data only}
-
- Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. New England Journal of Medicine 2000;343(13):898-904. - PubMed
Cheshmavar 2021 {published data only}
-
- Cheshmavar M, Mirmosayyeb O, Badihian N, Badihian S, Shaygannejad V. Rituximab and glatiramer acetate in secondary progressive multiple sclerosis: A randomized clinical trial. Acta Neurologica Scandinavica 2021;143(2):178-87. - PubMed
-
- NCT03315923. Comparison of clinical effects of rituximab and glatiramer acetate in secondary progressive multiple sclerosis patients [Comparison of expanded disability status scale, GAD-enhanced brain lesions, annualized relapse rate, and side effects between active secondary progressive multiple sclerosis patients on Rituximab and Glatiramer acetate]. clinicaltrials.gov/show/NCT03315923 (first received 20 October 2017).
CLARITY 2010 {published data only}
-
- Cook S, Leist T, Comi G, Montalban X, Giovannoni G, Nolting A, et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: An integrated analysis. Multiple Sclerosis and Related Disorders 2019;29:157-67. - PubMed
-
- Cook S, Leist T, Comi G, Montalban X, Sylvester E, Hicking C, et al. Infections during periods of grade 3 or 4 lymphopenia in patients taking CLADRIBINE tablets 3.5 mg/kg: Data from an integrated safety analysis. Multiple Sclerosis 2017;23(3):599.
-
- Cook S, Vermersch P, Comi G, Giovannoni G, Rammohan K, Rieckmann P, et al. Safety and tolerability of cladribine tablets in multiple sclerosis: the CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) study. Multiple Sclerosis (Houndmills, Basingstoke, England) 2011;17(5):578-93. - PubMed
-
- Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Soelberg Sørensen P, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. New England Journal of Medicine 2010;362(5):416-26. Appendix, p. 1-17. - PubMed
-
- NCT00213135. A safety and efficacy study of oral cladribine in subjects with relapsing-remitting multiple sclerosis (RRMS) (CLARITY) [A phase III, randomized, double-blind, three-arm, placebo-controlled, multi-center study to evaluate the safety and efficacy of oral Cladribine in subjects with relapsing-remitting multiple sclerosis (RRMS)]. clinicaltrials.gov/show/NCT00213135 (first received 21 September 2005).
CombiRx 2013 {published data only}
-
- NCT00211887. Combination therapy in patients with relapsing-remitting multiple sclerosis (MS) CombiRx [A multi-center, double-blind, randomized study comparing the combined use of Interferon beta-1a and Glatiramer acetate to either agent alone in patients with relapsing-remitting multiple sclerosis (CombiRx)]. clinicaltrials.gov/show/NCT00211887 (first received 21 September 2005).
Comi 2001 {published data only}
-
- Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging--measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Annals of Neurology 2001;49(3):290-7. - PubMed
Comi 2008 {published data only}
-
- Comi G, Pulizzi A, Rovaris M, Abramsky O, Arbizu T, Boiko A, et al. Effect of laquinimod on MRI-monitored disease activity in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 2008;371(9630):2085-92. - PubMed
-
- NCT00349193. A study to evaluate the effectiveness, tolerability and safety of laquinimod [A multinational, multicenter randomized double-blind, parallel-group, placebo-controlled study, to evaluate the efficacy, tolerability and safety of two doses of, oral Laquinimod in relapsing remitting (R-R) multiple sclerosis (MS) subjects]. clinicaltrials.gov/show/NCT00349193 (first received 6 July 2006).
CONCERTO 2022 {published data only}
-
- Comi G, Dadon Y, Sasson N, Steinerman JR, Knappertz V, Vollmer TL, et al. CONCERTO: a randomized, placebo-controlled trial of oral laquinimod in relapsing-remitting multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2022;28(4):608-19. - PubMed
-
- NCT01707992. The efficacy, safety, and tolerability of Laquinimod in participants with relapsing remitting multiple sclerosis (RRMS) (CONCERTO) [A multinational, multicenter, randomized, double-blind, parallel-group, placebo-controlled study followed by an active treatment period, to evaluate the efficacy, safety and tolerability of two doses of oral administration of Laquinimod (0.6 mg/day or 1.2 mg/day) in patients with relapsing remitting multiple sclerosis (RRMS)]. clinicaltrials.gov/show/NCT01707992 (first received 16 October 2012).
CONFIRM 2012 {published data only}
-
- Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. New England Journal of Medicine 2012;367(12):1087-97. Appendix, Protocol; p. 1-34. - PubMed
-
- NCT00451451. Efficacy and safety study of oral BG00012 with active reference in relapsing-remitting multiple sclerosis (CONFIRM) [A randomized, multicenter, placebo-controlled and active reference (Glatiramer acetate) comparison study to evaluate the efficacy and safety of BG00012 in subjects with relapsing-remitting multiple sclerosis]. clinicaltrials.gov/show/NCT00451451 (first received 23 March 2007).
DECIDE 2015 {published data only}
-
- Kappos L, Wiendl H, Selmaj K, Arnold DL, Havrdova E, Boyko A, et al. Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. New England Journal of Medicine 2015;373(15):1418-28. Supplementary Appendix; p. 1-36. - PubMed
-
- Krueger JG, Kircik L, Hougeir F, Friedman A, You X, Lucas N, et al. Cutaneous adverse events in the randomized, double-blind, active-comparator DECIDE study of daclizumab high-yield process versus intramuscular interferon beta-1a in relapsing-remitting multiple sclerosis. Advances in Therapy 2016;33(7):1231-45. - PMC - PubMed
-
- NCT01064401. Efficacy and safety of BIIB019 (daclizumab high yield process) versus interferon β 1a in participants with relapsing-remitting multiple sclerosis (DECIDE) [Multicenter, double-blind, randomized, parallel-group, monotherapy, active-control study to determine the efficacy and safety of Daclizumab high yield process (DAC HYP) versus Avonex® (interferon β 1a) in patients with relapsing-remitting multiple sclerosis]. clinicaltrials.gov/show/NCT01064401 (first received 8 February 2010).
DEFINE 2012 {published data only}
-
- Gold R, Arnold DL, Bar-Or A, Fox RJ, Kappos L, Chen C, et al. Safety and efficacy of delayed-release dimethyl fumarate in patients with relapsing-remitting multiple sclerosis: 9 years' follow-up of DEFINE, CONFIRM, and ENDORSE. Therapeutic Advances in Neurological Disorders 2020;13:1756286420915005. - PMC - PubMed
-
- Gold R, Giovannoni G, Phillips JT, Fox RJ, Yang L, Taylor C. Efficacy of delayed-release dimethyl FUMARATE in newly diagnosed patients with relapsing-remitting multiple sclerosis: Eight-year follow-up of an integrated analysis of DEFINE, CONFIRM, and ENDORSE. Multiple Sclerosis 2017;23(3):313-4.
-
- Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. New England Journal of Medicine 2012;367(12):1098-107. Appendix Protocol; p. 1-88. - PubMed
-
- NCT00420212. Efficacy and safety of oral BG00012 in relapsing-remitting multiple sclerosis (DEFINE) [A randomized, multicenter, double-blind, placebo-controlled, dose-comparison study to determine the efficacy and safety of BG00012 in subjects with relapsing-remitting multiple sclerosis]. clinicaltrials.gov/show/NCT00420212 (first received 11 January 2007).
Ellison 1989 {published data only}
-
- Ellison G, Myers L, Mickey M, Graves M, Tourtellotte W, Syndulko K, et al. A placebo-controlled, randomized, double-masked, variable dosage, clinical trial of azathioprine with and without methylprednisolone in multiple sclerosis. Neurology 1989;39(8):1018-26. - PubMed
Etemadifar 2006 {published data only}
-
- Etemadifar M, Janghorbani M, Shaygannejad V. Comparison of Betaferon, Avonex, and Rebif in treatment of relapsing-remitting multiple sclerosis. Acta Neurologica Scandinavica 2006;113(5):283-7. - PubMed
Etemadifar 2007 {published data only}
-
- Etemadifar M, Janghorbani M, Shaygannejad V. Comparison of interferon beta products and azathioprine in the treatment of relapsing-remitting multiple sclerosis. Journal of Neurology 2007;254(12):1723-8. - PubMed
ETOMS 2001 {published data only}
-
- Comi G, Filippi M, Barkhof F, Durelli L, Edan G, Fernández O, et al. Early Treatmentof Multiple Sclerosis Study Group. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet 2001;357(9268):1576-82. - PubMed
European Study Group 1998 {published data only}
-
- European SG. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive MS. Lancet 1998;352(9139):1491-7. - PubMed
-
- Kappos L, Polman C, Pozzilli C, Thompson A, Beckmann K, Dahlke F, European Study Group in Interferon beta-1b in Secondary-Progressive MS. Final analysis of the European multicenter trial on IFNbeta-1b in secondary-progressive MS. Neurology 2001;57(11):1969-75. - PubMed
EVIDENCE 2002 {published data only}
-
- Coyle PK, Reder AT, Freedman MS, Fang J, Dangond F. Early MRI results and odds of attaining 'no evidence of disease activity' status in MS patients treated with interferon β-1a in the EVIDENCE study. Journal of the Neurological Sciences 2017;379:151-6. - PubMed
-
- NCT00292266. A study of Rebif® compared with Avonex® in the treatment of relapsing-remitting multiple sclerosis (MS) [An open label, randomized, multicenter, comparative, parallel group study of Rebif® 44 mcg administered three times per week by subcutaneous injection, compared with Avonex® 30 mcg administered once per week by intramuscular injection in the treatment of relapsing-remitting multiple sclerosis]. ClinicalTrials.gov/show/NCT00292266 (first received 15 February 2006).
-
- Panitch H, Goodin DS, Francis G, Chang P, Coyle PK, O'Connor P, et al. Randomized, comparative study of interferon beta-1a treatment regimens in MS: The EVIDENCE Trial. Neurology 2002;59(10):1496-506. - PubMed
-
- Schwid S, Panitch H. Full results of the Evidence of Interferon Dose-Response-European North American Comparative Efficacy (EVIDENCE) study: a multicenter, randomized, assessor-blinded comparison of low-dose weekly versus high-dose, high-frequency interferon beta-1a for relapsing multiple sclerosis. Clinical Therapeutics 2007;29(9):2031-48. - PubMed
EVOLVE‐MS‐2 2020 {published data only}
-
- NCT03093324. A tolerability study of ALKS 8700 in subjects with relapsing remitting multiple sclerosis (RRMS) EVOLVE-MS-2 [A phase 3 study in subjects with relapsing remitting multiple sclerosis to evaluate the tolerability of ALKS 8700 and Dimethyl fumarate]. clinicaltrials.gov/show/NCT03093324 (first received 28 March 2017).
-
- Naismith RT, Wundes A, Ziemssen T, Jasinska E, Freedman MS, Lembo AJ, et al. Diroximel fumarate demonstrates an improved gastrointestinal tolerability profile compared with dimethyl fumarate in patients with relapsing-remitting multiple sclerosis: results from the randomized, double-blind, phase III EVOLVE-MS-2 study. CNS Drugs 2020;34(2):185-96. - PMC - PubMed
EXPAND 2018 {published data only}
-
- Kappos L, Bar-Or A, Cree BAC, Fox RJ, Giovannoni G, Gold R, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 2018;391(10127):1263-73. Supplementary appendix; p. 1-20. - PubMed
-
- NCT01665144. Exploring the efficacy and safety of siponimod in patients with secondary progressive multiple sclerosis (EXPAND) [A multicenter, randomized, double-blind, parallel-group, placebo-controlled variable treatment duration study evaluating the efficacy and safety of Siponimod (BAF312) in patients with secondary progressive multiple sclerosis followed by extended treatment with open-label BAF312]. clinicaltrials.gov/show/NCT01665144 (first received 15 August 2012).
Fazekas 1997 {published data only}
-
- Fazekas F, Deisenhammer F, Strasser-Fuchs S, Nahler G, Mamoli B. Randomised placebo-controlled trial of monthly intravenous immunoglobulin therapy in relapsing-remitting multiple sclerosis. Lancet 1997;349(9052):589-93. - PubMed
Fazekas 2008 {published data only}
-
- Fazekas F, Lublin F, Li D, Freedman M, Hartung H, Rieckmann P, et al. Intravenous immunoglobulin in relapsing-remitting multiple sclerosis: a dose-finding trial. Neurology 2008;71(4):265-71. - PubMed
FREEDOMS 2010 {published data only}
-
- Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. New England Journal of Medicine 2010;362(5):387-401. Supplementary Appendix; p. 1-9. - PubMed
-
- NCT00289978. Efficacy and safety of Fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS) [A 24-month, double-blind, randomized, multicenter, placebo-controlled, parallel-group study comparing the efficacy and safety of Fingolimod 1.25 mg and 0.5 mg administered orally once daily versus placebo in patients with relapsing-remitting multiple sclerosis]. clinicaltrials.gov/show/NCT00289978 (first received 10 February 2006).
FREEDOMS II 2014 {published data only}
-
- Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurology 2014;13(6):545-56. Supplementary webappendix; p. 1-12. - PubMed
-
- NCT00355134. Efficacy and safety of fingolimod (FTY720) in patients with relapsing-remitting multiple sclerosis (FREEDOMS II) [24-month double-blind, randomized, multicenter, placebo-controlled, parallel-group study comparing the efficacy and safety of 0.5 mg and 1.25 mg Fingolimod (FTY720) administered orally once daily versus placebo in patients with relapsing-remitting multiple sclerosis with optional extension phase]. clinicaltrials.gov/show/NCT00355134 (first received 21 July 2006).
-
- Radue EW, Sprenger T, Vera A, Francis G, Rochotte E, Tomic D, et al. Effect of fingolimod on evolution of baseline enhancing MRI lesions into persistent T1 hypointense lesions: Post hoc analysis of the FREEDOMS study. Multiple Sclerosis 2014;20(1):112-3.
FUMAPMS 2021 {published data only}
-
- NCT02959658. Dimethyl fumarate treatment of primary progressive multiple sclerosis (FUMAPMS) [Dimethyl Fumarate treatment of primary progressive multiple sclerosis]. clinicaltrials.gov/show/NCT02959658 (first received 9 November 2016).
GALA 2013 {published data only}
-
- Khan O, Rieckmann P, Boyko A, Selmaj K, Zivadinov R, GALA Study Group. Three times weekly glatiramer acetate in relapsing-remitting multiple sclerosis. Annals of Neurology 2013;73(6):705-13. Supplementary Table 3; Serious Adverse Events by System Organ Class, p.3. - PubMed
GATE 2015 {published data only}
-
- Cohen J, Belova A, Selmaj K, Wolf C, Sormani MP, Oberyé J, et al. Equivalence of generic glatiramer acetate in multiple sclerosis: a randomized clinical trial. JAMA Neurology 2015;72(12):1433-41. Appendix 1, Protocol; p. 1-64. Appendix 2, [eMethods; eTables 1-5; eFigure]. - PubMed
-
- Cohen J, Belova A, Selmaj K, Wolf C, Sormani MP, Oberyé J, et al. Multi-centre, randomized, double-blind, placebo-controlled, parallel-group, 9 month, equivalence trial comparing the efficacy and safety and tolerability of GTR (Synthon BV) to Copaxone® (Teva) in subjects with relapsing remitting multiple sclerosis followed by an open-label 15 month GTR treatment part evaluating the long-term GTR treatment effects. JAMA Neurology 2015;72(12):1433-41. Clinical trial protocol (version 4.0); p. 1-64.
-
- NCT01489254. Efficacy and safety of GTR in comparison to Copaxone® (GATE) [Multi-centre, randomized, double-blind, placebo-controlled, parallel-group, 9 month, equivalence trial comparing the efficacy and safety and tolerability of GTR (Synthon BV) to Copaxone® (Teva) in subjects with relapsing remitting multiple sclerosis followed by an open-label 15 month GTR treatment part evaluating the long-term GTR treatment]. clinicaltrials.gov/show/NCT01489254 (first received 9 December 2011).
Ghezzi 1989 {published data only}
-
- Ghezzi A, Di Falco M, Locatelli C. Clinical controlled randomized trial of azathioprine in multiple sclerosis. Elsevier, 1989.
GOLDEN 2017 {published data only}
-
- Comi G, Patti F, Rocca MA, Mattioli FC, Amato MP, Gallo P, et al. Efficacy of fingolimod and interferon beta-1b on cognitive, MRI, and clinical outcomes in relapsing-remitting multiple sclerosis: an 18-month,open-label, rater-blinded, randomised, multicentre study (the GOLDEN study). Journal of Neurology 2017;264(12):2436-49. - PMC - PubMed
-
- NCT01333501. Fingolimod versus interferon beta 1b in cognitive symptoms (cognition) [A 18-month, open-label, rater-blinded, randomized, multi-center, active-controlled, parallel-group pilot study to assess efficacy and safety of Fingolimod in comparison to Interferon beta 1b in treating the cognitive symptoms associated to relapsing-remitting multiple sclerosis and to assess possible relationship of these effects to regional brain atrophy]. clinicaltrials.gov/show/NCT01333501 (first received 12 April 2011).
Goodkin 1991 {published data only}
-
- Goodkin D, Bailly R, Teetzen M, Hertsgaard D, Beatty W. The efficacy of azathioprine in relapsing-remitting multiple sclerosis. Neurology 1991;41:20-5. - PubMed
Goodkin 1995 {published data only}
-
- Goodkin D, Rudick R, VanderBrug Medendorp S, Daughtry M, Schwetz K, Fischer J, et al. Low-dose (7.5 mg) oral methotrexate reduces the rate of progression in chronic progressive multiple sclerosis. Annals of Neurology 1995;37(1):30-40. - PubMed
Hartung 2002 {published data only}
-
- Hartung H, Gonsette R, Konig N, Kwiecinski H, Guseo A, Morrissey S, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet 2002;360(9350):2018-25. - PubMed
Hauser 2008 {published data only}
-
- Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. New England Journal of Medicine 2008;358(7):676-88. - PubMed
-
- NCT00097188. A study to evaluate rituximab in adults with relapsing remitting multiple sclerosis [A phase II, randomized, double-blind, parallel-group, placebo-controlled, multicenter study to evaluate the safety and efficacy of Rituximab (Mabthera/Rituxan) in adults with relapsing remitting multiple sclerosis]. clinicaltrials.gov/show/NCT00097188 (first received 19 November 2004).
Hommes 2004 {published data only}
-
- Hommes O, Sorensen P, Fazekas F, Enriquez M, Koelmel H, Fernandez O, et al. Intravenous immunoglobulin in secondary progressive multiple sclerosis: randomised placebo-controlled trial. Lancet 2004;364(9440):1149-56. - PubMed
-
- Hommes OR, Maas-Enriquez M. ESIMS--an ongoing clinical trial in secondary progressive multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2000;6(Suppl 2):S27-32. - PubMed
IFNB MS Group 1993 {published data only}
-
- IFNB MSG. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology 1993;43(4):655-61. - PubMed
IMPACT 2002 {published data only}
-
- Cohen J, Cutter G, Fischer J, Goodman A, Heidenreich F, Kooijmans M, et al. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology 2002;59(5):679-87. - PubMed
IMPROVE 2010 {published data only}
-
- De Stefano N, Curtin F, Stubinski B, Blevins G, Drulovic J, Issard D, et al. Rapid benefits of a new formulation of subcutaneous interferon beta-1a in relapsing-remitting multiple sclerosis. Multiple Sclerosis 2010;16(7):888-92. - PubMed
-
- NCT00441103. A study to evaluate Rebif® new formulation (interferon-beta-1a) in relapsing remitting multiple sclerosis (IMPROVE) [A two-arm, randomized, double-blind, control group-compared, multicenter, phase IIIB study with monthly MRI and biomarker assessments to evaluate the efficacy, safety, and tolerability of Rebif® new formulation (IFN beta-1a) in subjects with relapsing remitting multiple sclerosis]. clinicaltrials.gov/show/NCT00441103 (first received 28 February 2007).
INCOMIN 2002 {published data only}
-
- Durelli L, Verdun E, Barbero P, Bergui M, Versino E, Ghezzi A, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet 2002;359:1453-60. - PubMed
INFORMS 2016 {published data only}
-
- Fox E, Lublin F, Wolinsky J, Cohen J, Meng X, Ziehn M, et al. Analysis of lymphocyte counts and infection rates with fingolimod in patients with primary progressive multiple sclerosis over the INFORMS trial. Neurology 2018;90(15 Supplement):P1.387.
-
- Lublin F, Miller DH, Freedman MS, Cree BA, Wolinsky JS, Weiner H, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2016;387(10023):1075-84. Supplementary appendix; p. 1-17. - PubMed
-
- NCT00731692. This was an open-label, single-arm extension study (CFTY720D2306E1) to a double-blind, randomized multicenter, placebo-controlled, parallel-group core study (CFTY720D2306) in PPMS. (INFORMS) [A double-blind, randomized, multicenter, placebo-controlled, parallel-group study comparing the efficacy and safety of 0.5mg Fingolimod administered orally once daily versus placebo in patients with primary progressive multiple sclerosis and an open-label, single-arm extension study to the double-blind, randomized, multicenter, placebo-controlled, parallel-group study comparing the efficacy and safety of 0.5 mg FTY720 administered orally once daily versus placebo in patients with primary progressive multiple sclerosis]. clinicaltrials.gov/show/NCT00731692 (first received 11 August 2008).
Johnson 1995 {published data only}
-
- Johnson K, Brooks B, Cohen J, Ford C, Goldstein J, Lisak R, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995;45(7):1268-76. - PubMed
-
- Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. 1998 [classical article]. Neurology 2001;57(12 Suppl 5):S46-53. - PubMed
-
- Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Copolymer 1 Multiple Sclerosis Study Group. Neurology 1998;50(3):701-8. - PubMed
Kappos 2006 {published data only}
-
- Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. New England Journal of Medicine 2006;355(11):1124-40. Supplementary Appendix; p. 1. - PubMed
-
- NCT00333138. Efficacy and safety of FTY720 in patients with relapsing multiple sclerosis [Double-blind, randomized, placebo-controlled, parallel-group, multicenter study evaluating the safety, tolerability and effect on MRI lesion parameters of FTY720 vs placebo in patients with relapsing multiple sclerosis including 18-month extension phase]. clinicaltrials.gov/show/NCT00333138 (first received 2 June 2006).
Kappos 2008 {published data only}
-
- Kappos L, Gold R, Miller DH, Macmanus DG, Havrdova E, Limmroth V, et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 2008;372(9648):1463-72. - PubMed
-
- NCT00168701. Efficacy and safety of BG00012 in MS [Double-blind, placebo-controlled, dose-ranging study to determine the efficacy and safety of BG00012 in subjects with relapsing-remitting multiple sclerosis]. ClinicalTrials.gov/show/ NCT00168701 (first received 15 September 2005).
Kappos 2011 {published data only}
-
- Kappos L, Li D, Calabresi PA, O'Connor P, Bar-Or A, Barkhof F, et al. Ocrelizumab in relapsing-remitting multiple sclerosis:a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011;378(9805):1779-87. - PubMed
-
- NCT00676715. A study of the efficacy and safety of ocrelizumab in patients with relapsing-remitting multiple sclerosis [Phase II, multicenter, randomized, parallel-group, partially blinded, placebo and Avonex controlled dose finding study to evaluate the efficacy as measured by brain MRI lesions, and safety of 2 dose regimens of Ocrelizumab in patients with RRMS]. clinicaltrials.gov/show/NCT00676715 (first received 13 May 2008).
Knobler 1993 {published data only}
-
- Knobler R, Greenstein J, Johnson K, Lublin F, Panitch H, Conway K, et al. Systemic recombinant human interferon-beta treatment of relapsing-remitting multiple sclerosis: pilot study analysis and six-year follow-up. Journal of Interferon & Cytokine Research 1993;13:333-40. - PubMed
Koch‐Henriksen 2006 {published data only}
-
- Koch-Henriksen N, Sørensen P, Christensen T, Frederiksen J, Ravnborg M, Jensen K, et al. A randomised study of two interferon-beta treatments in relapsing-remitting multiple sclerosis. Neurology 2006;66(7):1056-60. - PubMed
Leary 2003 {published data only}
-
- Leary S, Miller D, Stevenson V, Brex P, Chard D, Thompson A. Interferon beta-1a in primary progressive MS: an exploratory, randomized, controlled trial. Neurology 2003;60(1):44-51. - PubMed
Lewanska 2002 {published data only}
-
- Lewanska M, Siger-Zajdel M, Selmaj K. No difference in efficacy of two different doses of intravenous immunoglobulins in MS: clinical and MRI assessment. European Journal of Neurology 2002;9(6):565-72. - PubMed
Likosky 1991 {published data only}
MAIN TRIAL 2014 {published data only}
-
- EudraCT 2006-004937-13. Multicentee randomized controlled study of azathioprine versus iterferon beta in relapsing remitting multiple sclerosis. www.clinicaltrialsregister.eu/ctr-search/search?query=2006-004937-13 (first received 17 August 2006).
Masjedi 2021 {published data only}
Milanese 1993 {published data only}
-
- Milanese C, La Mantia L, Salmaggi A, Eoli M. A double blind study on azathioprine efficacy in multiple sclerosis: final report. Journal of Neurology 1993;240(5):295-8. - PubMed
Millefiorini 1997 {published data only}
-
- Millefiorini E, Gasperini C, Pozzilli C, D'Andrea F, Bastianello S, Trojano M, et al. Randomized placebo-controlled trial of mitoxantrone in relapsing-remitting multiple sclerosis: 24-month clinical and MRI outcome. Journal of Neurology 1997;244(3):153-9. - PubMed
Miller 1961 {published data only}
-
- Miller H, Newell D, Ridley A. Multiple sclerosis. Trials of maintenance treatment with prednisolone and soluble aspirin. Lancet 1961;1(7169):127-9. - PubMed
Miller 2003 {published data only}
-
- Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. New England Journal of Medicine 2003;348(1):15-23. - PubMed
MIRROR 2018 {published data only}
-
- Bar-Or A, Grove RA, Austin DJ, Tolson JM, VanMeter SA, Lewis EW, et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study. Neurology 2018;90(20):e1805-14. e-supplement; links.lww.com/WNL/A437. - PMC - PubMed
-
- NCT01457924. Ofatumumab subcutaneous administration in subjects with relapsing-remitting multiple sclerosis (MIRROR) [A randomized, double-blind, placebo-controlled, parallel-group, dose-ranging study to investigate the MRI efficacy and safety of six months' administration of Ofatumumab in subjects with relapsing-remitting multiple sclerosis (RRMS)]. clinicaltrials.gov/show/NCT01457924 (first received 24 October 2011).
-
- Sorenson PS, Kavanagh ST, Austin DJ, Grove RA, Lopez MC, Tolson JM, et al. Follow-up data from the Mirror study: A dose-ranging study of subcutaneous ofatumumab in subjects with relapsing-remitting multiple sclerosis. Multiple Sclerosis 2014;20(1):P048.
Mokhber 2014 {published data only}
-
- Mokhber N, Azarpazhooh A, Orouji E, Rao SM, Khorram B, Sahraian MA, et al. Cognitive dysfunction in patients with multiple sclerosis treated with different types of interferon beta: A randomized clinical trial. Journal of the Neurological Sciences 2014;342(1-2):16-20. - PubMed
Montalban 2009 {published data only}
-
- Montalban X, Sastre-Garriga J, Tintore M, Brieva L, Aymerich F, Rio J, et al. A single-center, randomized, double-blind, placebo-controlled study of interferon beta-1b on primary progressive and transitional multiple sclerosis. Multiple Sclerosis 2009;15(10):1195-205. - PubMed
Motamed 2007 {published data only}
-
- Motamed MR, Najimi N, Fereshtehnejad SM. The effect of interferon-beta1a on relapses and progression of disability in patients with clinically isolate syndromes (CIS) suggestive of multiple sclerosis. Clinical Neurology and Neurosurgery 2007;109(4):344-9. - PubMed
MOVING 2020 {published data only}
-
- Albert C, Mikolajczak J, Liekfeld A, Piper SK, Scheel M, Zimmermann HG, et al. Fingolimod after a first unilateral episode of acute optic neuritis (MOVING) - preliminary results from a randomized, rater-blind, active-controlled, phase 2 trial. BMC Neurology 2020;20(1):75. 2020;20(1):75. Additional file 1 Supplementary methods and results; p. 1-14. - PMC - PubMed
-
- NCT01647880. MOdification of VIsual Outcomes after Optic Neuritis in CIS or MS by Gilenya (MOVING Study) [Phase II/III study to investigate the effects of Fingolimod versus Interferon beta-1b on visual recovery after optic neuritis]. clinicaltrials.gov/show/NCT01647880 (first received 24 July 2012).
MSCRG 1996 {published data only}
-
- Jacobs L, Cookfair D, Rudick R, Herndon R, Richert J, Salazar A, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Annals of Neurology 1996;39:285-94. - PubMed
NASP 2004 {published data only}
-
- Panitch H, Miller A, Paty D, Weinshenker B. Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology 2004;63(10):1788-95. - PubMed
Noseworthy 2000 {published data only}
-
- Noseworthy JH, O'Brien PC, Weinshenker BG, Weis JA, Petterson TM, Erickson BJ, et al. IV immunoglobulin does not reverse established weakness in MS. Neurology 2000;55(8):1135-43. - PubMed
O'Connor 2006 {published data only}
-
- O'Connor PW, Li D, Freedman MS, Bar-Or A, Rice GP, Confavreux C, et al. A Phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 2006;66(6):894-900. - PubMed
OLYMPUS 2009 {published data only}
-
- Hawker K, O'Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Annals of Neurology 2009;66(4):460-71. - PubMed
-
- NCT00087529. A study to evaluate the safety and efficacy of rituximab in adults with primary progressive multiple sclerosis (OLYMPUS) [A phase II/III, randomized, double-blind, parallel-group, placebo-controlled, multicenter study to evaluate the safety and efficacy of Rituximab in adults with primary progressive multiple sclerosis]. clinicaltrials.gov/show/NCT00087529 (first received 13 July 2004).
-
- Zhang J, Waubant E, Cutter G, Wolinsky J, Glanzman R. EDSS variability before randomization may limit treatment discovery in primary progressive MS. Multiple Sclerosis 2013;19(6):775-81. - PubMed
OPERA I 2017 {published data only}
-
- De Seze J, Hauser SL, Kappos L, Montalban X, Pozzilli C, Chognot C, et al. Infusion-related reactions with OCRELIZUMAB in phase III studies. Multiple Sclerosis 2017;23(3):878-9.
-
- Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus Interferon beta-1a in relapsing multiple sclerosis. New England Journal of Medicine 2017;376(3):221-34. Supplementary appendix; p. 1-36. - PubMed
-
- NCT01247324. A study of ocrelizumab in comparison with interferon beta-1a (Rebif) in participants with relapsing multiple sclerosis [A randomized, double-blind, double-dummy, parallel-group study to evaluate the efficacy and safety of Ocrelizumab in comparison to Interferon beta-1a (Rebif®) in patients with relapsing multiple sclerosis]. clinicaltrials.gov/show/NCT01247324 (first received 24 November 2010).
OPERA II 2017 {published data only}
-
- Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. New England Journal of Medicine 2017;376(3):221-34. - PubMed
-
- NCT01412333. A study of ocrelizumab in comparison with interferon beta-1a (Rebif) in participants with relapsing multiple sclerosis [A randomized, double-blind, double-dummy, parallel-group study to evaluate the efficacy and safety of Ocrelizumab in comparison to Interferon beta-1a (Rebif) in patients with relapsing multiple sclerosis]. clinicaltrials.gov/show/NCT01412333 (first received 9 August 2011).
ORACLE 2014 {published data only}
-
- Leist T, Comi G, Cree B, Coyle P, Freedman M, Hartung H, et al. Oral cladribine delays time to conversion to clinically definite MS in patients with a first demyelinating event: top line results from the phase III ORACLE MS Study. Neurology 2013;80(7 Supplement):P07.114. - PubMed
-
- Leist TP, Comi G, Cree BA, Coyle PK, Freedman MS, Hartung HP, et al. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurology 2014;13(3):257-67. - PubMed
-
- NCT00725985. Oral cladribine in early multiple sclerosis (MS) (ORACLE MS) [A phase III, randomized, double-blind, placebo-controlled, multi-center clinical trial of oral Cladribine in subjects with a first clinical event at high risk of converting to MS]. clinicaltrials.gov/show/NCT00725985 (first received 31 July 2008).
ORATORIO 2017 {published data only}
-
- Fox EJ, Markowitz C, Applebee A, Montalban X, Wolinsky JS, Belachew S, et al. Effect of OCRELIZUMAB on upper limb function in patients with primary progressive multiple sclerosis in the ORATORIO study. Multiple Sclerosis 2017;23(3):658-9.
-
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. New England Journal of Medicine 2017;376(3):209-20. Supplementary appendix; p. 1-27. - PubMed
-
- NCT01194570. A study of Ocrelizumab in participants with primary progressive multiple sclerosis [A phase III, multicentre, randomized, parallel-group, double-blind, placebo controlled study to evaluate the efficacy and safety of Ocrelizumab in adults with primary progressive multiple sclerosis]. clinicaltrials.gov/show/NCT01194570 (first received 3 September 2010).
OWIMS 1999 {published data only}
-
- OWIMS. Evidence of interferon beta-1a dose response in relapsing-remitting MS: the OWIMS Study. The Once Weekly Interferon for MS Study Group. Neurology 1999;53(4):679-86. - PubMed
Pakdaman 2007 {published data only}
-
- Pakdaman H, Sahraian MA, Fallah A, Pakdaman R, Ghareghozli K, Ghafarpour M, et al. Effect of early interferon beta-1a therapy on conversion to multiple sclerosis in Iranian patients with a first demyelinating event. Acta Neurologica Scandinavica 2007;115(6):429-31. - PubMed
Pohlau 2007 {published data only}
-
- Pohlau D, Przuntek H, Sailer M, Bethke F, Koehler J, Konig N, et al. Intravenous immunoglobulin in primary and secondary chronic progressive multiple sclerosis: a randomized placebo controlled multicentre study. Multiple Sclerosis 2007;13(9):1107-17. - PubMed
Polman 2005 {published data only}
-
- Polman C, Barkhof F, Sandberg-Wollheim M, Linde A, Nordle O, Nederman T, et al. Treatment with laquinimod reduces development of active MRI lesions in relapsing MS. Neurology 2005;64(6):987-91. - PubMed
PreCISe 2009 {published data only}
-
- Comi G, Martinelli V, Rodegher M, Moiola L, Bajenaru O, Carra A, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet 2009;374(9700):1503-11. - PubMed
-
- NCT00666224. Evaluate early glatiramer acetate treatment in delaying conversion to clinically definite multiple sclerosis of subjects presenting with clinically isolated syndrome (PreCISe) [A multinational, multicenter, randomized, double-blind, placebo controlled, parallel group study to evaluate the effect of early Glatiramer acetate treatment in delaying the conversion to clinically definite multiple sclerosis (CDMS) of subjects presenting with clinically isolated syndrome (CIS)]. clinicaltrials.gov/show/NCT00666224 (first received 24 April 2008).
PRISMS 1998 {published data only}
-
- PRISMS. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 1998;352(9139):1498-504. - PubMed
PROMESS 2017 {published data only}
-
- Brochet B, Deloire MS, Perez P, Loock T, Baschet L, Debouverie M, et al. Double-blind controlled randomized trial of cyclophosphamide versus methylprednisolone in secondary progressive multiple sclerosis. PLoS One 2017;12(1):e0168834. Protocol synopsys; S2 File. CONSORT 2010 checklist promess; S4 File. - PMC - PubMed
-
- NCT00241254. Efficacy of cyclophosphamide versus methylprednisolone in patients with secondary progressive multiple sclerosis (PROMESS) [A double-blind, two-arm, multicenter, randomized trial to evaluate efficacy of Cyclophosphamide versus Methylprednisolone in patients with recent secondary progressive multiple sclerosis: P.R.OM.E.S.S study]. clinicaltrials.gov/show/NCT00241254 (first received 18 October 2005).
RADIANCE 2019 {published data only}
-
- Cohen J, Comi G, Selmaj K, Bar-Or A, Arnold D, Steinman L, et al. Clinical and magnetic resonance imaging results from Radiance Part B, a multicenter, randomized, double-blind, phase 3 trial of Ozanimod versus intramuscular Interferon β-1a in relapsing multiple sclerosis (RMS). Neurology 2018;90(15 Supplement):P3.410.
-
- Cohen JA, Comi G, Selmaj KW, Bar-Or A, Arnold DL, Steinman L, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurology 2019;18(11):1021-33. Supplementary appendix; p. 1-32. - PubMed
-
- NCT02047734. Efficacy and safety study of ozanimod in relapsing multiple sclerosis (RADIANCE) [A phase 2/3, multi-center, randomized, double-blind, placebo-controlled (part a) and double-blind, double-dummy, active-controlled (part b), parallel group study to evaluate the efficacy and safety of RPC1063 administered orally to relapsing multiple sclerosis patients]. clinicaltrials.gov/show/NCT02047734 (first received 28 January 2014).
REFLEX 2012 {published data only}
-
- Comi G, De Stefano N, Freedman MS, Barkhof F, Polman CH, Uitdehaag BM, et al. Comparison of two dosing frequencies of subcutaneous interferon beta-1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): a phase 3 randomised controlled trial. Lancet Neurology 2012;11(1):33-41. - PubMed
-
- NCT00404352. REbif FLEXible dosing in early multiple sclerosis (MS) (REFLEX) [A phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial of Rebif new formulation (44 microgram [mcg] three times weekly [tiw] and 44 mcg once weekly [ow]) in subjects at high risk of converting to multiple sclerosis (REFLEX)]. clinicaltrials.gov/show/NCT00404352 (first received 28 November 2006).
REFORMS 2012 {published data only}
-
- NCT00428584. RNF and Betaseron® Tolerability Study (REFORMS) [A randomized, multicenter, two arm, open label, twelve week phase IIIB study to evaluate the tolerability of Rebif (new formulation) (IFN beta-1a) and Betaseron (IFN beta-1b) in IFN-naive subjects with relapsing remitting multiple sclerosis (RRMS) followed by a single arm, eighty-two week minimum, Rebif (new formulation) only safety extension]. clinicaltrials.gov/show/NCT00428584 (first received 30 January 2007).
-
- Singer B, Bandari D, Cascione M, LaGanke C, Huddlestone J, Bennett R, et al. Comparative injection-site pain and tolerability of subcutaneous serum-free formulation of interferonβ-1a versus subcutaneous interferonβ-1b: results of the randomized, multicenter, Phase IIIb REFORMS study. BMC Neurology 2012;12:154. - PMC - PubMed
REGARD 2008 {published data only}
-
- Mikol D, Barkhof F, Chang P, Coyle P, Jeffery D, Schwid S, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurology 2008;7(10):903-14. - PubMed
-
- NCT00078338. Rebif® versus Copaxone® in the treatment of relapsing remitting multiple sclerosis [Phase IV, multicenter, open label, randomized study of Rebif® 44 mcg administered three times per week by subcutaneous injection compared with Copaxone® 20 mg administered daily by subcutaneous injection in the treatment of relapsing remitting multiple sclerosis]. clinicaltrials.gov/show/NCT00078338 (first received 26 February 2004).
REVEAL 2020 {published data only}
-
- Butzkueven H, Licata S, Jeffery D, Arnold DL, Filippi M, Geurts JJ, et al. Natalizumab versus fingolimod for patients with active relapsing-remitting multiple sclerosis: results from REVEAL, a prospective, randomised head-to-head study. British Medical Journal Open 2020;10(10):e038861. Online supplementary figure 1; Patient flow. - PMC - PubMed
-
- NCT02342704. Impact of Natalizumab versus Fingolimod in relapsing-remitting multiple sclerosis (RRMS) participants (REVEAL) [A multicenter, randomized, open-label study to assess the impact of Natalizumab versus Fingolimod on central nervous system tissue damage and recovery in active relapsing-remitting multiple sclerosis subjects]. clinicaltrials.gov/show/NCT02342704 (first received 21 January 2015).
Saida 2012 {published data only}
-
- NCT00537082. Efficacy and safety of FTY720 in patients with relapsing multiple sclerosis (MS) [A 6-month, double-blind, randomized, placebo-controlled, parallel-group, multicenter study comparing efficacy and safety of FTY720 0.5 mg and 1.25 mg administered orally once daily in patients with relapsing multiple sclerosis]. clinicaltrials.gov/show/NCT00537082 (first received 28 September 2007).
-
- Saida T, Kikuchi S, Itoyama Y, Hao Q, Kurosawa T, Nagato K, et al. A randomized, controlled trial of fingolimod (FTY720) in Japanese patients with multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2012;18(9):1269-77. Online appendix; p. 1-14. - PubMed
Saida 2017 {published data only}
-
- NCT01440101. Natalizumab (BG00002, Tysabri) study in Japanese participants with relapsing-remitting multiple sclerosis (RRMS) (Tysabri Japan) [Multicenter study of BG00002 in Japanese subjects with RRMS, consisting of a multiple-dose, open-label evaluation of its safety, tolerability, pharmacokinetics and pharmacodynamics (part a) and a randomized, double-blind, placebo-controlled, multiple-dose evaluation of safety and efficacy (part b)]. clinicaltrials.gov/show/NCT01440101 (first received 26 September 2011).
-
- Saida T, Kira JI, Kishida S, Yamamura T, Sudo Y, Ogiwara K, et al. Efficacy, safety, and pharmacokinetics of natalizumab in Japanese multiple sclerosis patients: A double-blind, randomized controlled trial and open-label pharmacokinetic study. Multiple Sclerosis and Related Disorders 2017;11:25-31. - PubMed
SELECT 2013 {published data only}
-
- Gold R, Giovannoni G, Selmaj K, Havrdova E, Montalban X, Radue EW, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet 2013;381(9884):2167-75. Supplementary appendix; p. 1-6. - PubMed
-
- NCT00390221. Safety and efficacy study of daclizumab high yield process (DAC HYP) to treat relapsing-remitting multiple sclerosis (SELECT) [Multicenter, double-blind, placebo-controlled, dose-ranging study to determine the safety and efficacy of Daclizumab HYP (DAC HYP) as a monotherapy treatment in subjects with relapsing-remitting multiple sclerosis]. clinicaltrials.gov/show/NCT00390221 (first received 19 October 2006).
-
- Phillips G, Guo S, Bender R, Havrdova E, Proskorovsky I, Vollmer T. Assessing the impact of multiple sclerosis disease activity and DACLIZUMAB HYP treatment on patient-reported outcomes: Results from the SELECT trial. Multiple Sclerosis and Related Disorders 2016;6:66-72. - PubMed
SPECTRIMS 2001 {published data only}
-
- SPECTRIMS. Randomized controlled trial of interferon- beta-1a in secondary progressive MS: Clinical results. Neurology 2001;56(11):1496-504. - PubMed
SUNBEAM 2019 {published data only}
-
- Comi G, Arnold D, Cree B, Kappos L, Selmaj K, Bar-Or A, et al. Ozanimod demonstrates efficacy and safety in a multicenter, randomized, double-blind, double-dummy, active-controlled phase 3 trial of relapsing multiple sclerosis (SUNBEAM). Neurology 2018;90(15 Supplement):P3.396.
-
- Comi G, Kappos L, Selmaj KW, Bar-Or A, Arnold DL, Steinman L, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurology 2019;18(11):1009-20. Supplementary appendix; p. 1-26. - PubMed
-
- Cree B, Bar-Or A, Comi G, Selmaj K, Arnold D, Steinman L, et al. Safety of Ozanimod versus Interferon β-1a in two multicenter, randomized, double-blind, parallel-group, active-controlled, double-dummy phase 3 studies in relapsing multiple sclerosis (SUNBEAM and RADIANCE Part B). Neurology 2018;90(15 Supplement):S36.006.
-
- NCT02294058. Study of ozanimod (RPC1063) in relapsing multiple sclerosis (MS) (SUNBEAM) [A phase 3, multi-center, randomized, double-blind, double-dummy, active controlled, parallel group study to evaluate the efficacy and safety of RPC1063 administered orally to relapsing multiple sclerosis patients]. clinicaltrials.gov/show/NCT02294058 (first received 19 November 2014).
TEMSO 2011 {published data only}
-
- NCT00134563. Study of teriflunomide in reducing the frequency of relapses and accumulation of disability in patients with multiple sclerosis (TEMSO) [A randomized, double-blind, placebo-controlled, parallel group design study to evaluate the efficacy and safety of teriflunomide in reducing the frequency of relapses and delaying the accumulation of physical disability in subjects with multiple sclerosis with relapses]. ClinicalTrials.gov/show/NCT00134563 (first received 25 August 2005).
-
- O'Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. New England Journal of Medicine 2011;365(14):1293-303. - PubMed
-
- Sprenger T, Kappos L, Radue EW, Gaetano L, Mueller-Lenke N, Wuerfel J, et al. Teriflunomide significantly slows brain volume loss in MS patients irrespective of disability progression. Neurology 2016;86(Suppl 1):16.
TENERE 2014 {published data only}
-
- NCT00883337. A study comparing the effectiveness and safety of teriflunomide and interferon beta-1a in patients with relapsing multiple sclerosis (TENERE) [A multi-center, randomized, parallel-group, rater-blinded study comparing the effectiveness and safety of Teriflunomide and Interferon beta-1a in patients with relapsing multiple sclerosis plus a long term extension period]. clinicaltrials.gov/show/NCT00883337 (first received17 April 2009).
-
- Vermersch P, Czlonkowska A, Grimaldi LM, Confavreux C, Comi G, Kappos L, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Multiple Sclerosis 2014;20(6):705-16. - PubMed
TOPIC 2014 {published data only}
-
- Miller AE, Wolinsky JS, Kappos L, Comi G, Freedman MS, Olsson TP, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurology 2014;13(10):977-86. Supplementary webappendix; p. 1-14. - PubMed
-
- NCT00622700. Phase III study with Teriflunomide versus placebo in patients with first clinical symptom of multiple sclerosis (TOPIC) [An international, multi-center, randomized, double-blind, placebo-controlled, parallel group study to evaluate the efficacy and safety of two year treatment with Teriflunomide 7 mg once daily and 14 mg once daily versus placebo in patients with a first clinical episode suggestive of multiple sclerosis plus a long term extension period]. clinicaltrials.gov/show/NCT00622700 (first received 25 February 2008).
TOWER 2014 {published data only}
-
- Confavreux C, O'Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurology 2014;13(3):247-56. - PubMed
-
- Freedman MS, Morawski J, Thangavelu K. Clinical efficacy of Teriflunomide over a fixed 2-year duration in the TOWER study [A multi-center double-blind parallel-group placebo-controlled study of the efficacy and safety of Teriflunomide in patients with relapsing multiple sclerosis]. Multiple Sclerosis Journal – Experimental, Translational and Clinical 2018;4(2):2055217318775236. - PMC - PubMed
-
- Miller AE, Xu X, Macdonell R, Vucic S, Truffinet P, Benamor M, et al. Efficacy and safety of teriflunomide in Asian patients with relapsing forms of multiple sclerosis: a subgroup analysis of the phase 3 TOWER study. Journal of Clinical Neuroscience 2019;59:229-31. - PubMed
-
- NCT00751881. An efficacy study of Teriflunomide in participants with relapsing multiple sclerosis (TOWER) [A multi-center double-blind parallel-group placebo-controlled study of the efficacy and safety of Teriflunomide in patients with relapsing multiple sclerosis]. clinicaltrials.gov/show/NCT00751881 (first received 12 September 2008).
TRANSFORMS 2010 {published data only}
-
- Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. New England Journal of Medicine 2010;362(5):402-15. Appendix, p. 1-11. - PubMed
-
- NCT00340834. Efficacy and safety of fingolimod in patients with relapsing-remitting multiple sclerosis with optional extension phase (TRANSFORMS) [A 12-month double-blind, randomized, multicenter, active-controlled, parallel-group study comparing the efficacy and safety of 0.5 mg and 1.25 mg Fingolimod (FTY720) administered orally once daily versus Interferon ß-1a (Avonex) administered im once weekly in patients with relapsing-remitting multiple sclerosis with optional extension]. clinicaltrials.gov/show/NCT00340834 (first received 21 June 2006).
Tubridy 1999 {published data only}
-
- Tubridy N, Behan PO, Capildeo R, Chaudhuri A, Forbes R, Hawkins CP, et al. The effect of anti-alpha4 integrin antibody on brain lesion activity in MS. The UK Antegren Study Group. Neurology 1999;53(3):466-72. - PubMed
Van de Wyngaert 2001 {published data only}
-
- de Wyngaert FA, Beguin C, D'Hooghe MB, Dooms G, Lissoir F, Carton H, et al. A double-blind clinical trial of mitoxantrone versus methylprednisolone in relapsing, secondary progressive multiple sclerosis. Acta Neurologica Belgica 2001;101(4):210-6. - PubMed
Wolinsky 2007 {published data only}
-
- Wolinsky J, Narayana P, O'Connor P, Coyle P, Ford C, Johnson K, et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Annals of Neurology 2007;61(1):14-24. - PubMed
Ziemssen 2017 {published data only}
-
- EudraCT: 2009-011234-99. A Phase I, sequential group, randomized, double-blind, placebo-controlled study to assess the tolerability and safety of escalating doses of oral laquinimod administereddaily in subjects with relapsing remitting multiple sclerosis (RRMS). www.clinicaltrialsregister.eu/ctr-search/trial/2009-011234-99/DE Registered 23 June 2009.
-
- Ziemssen T, Tumani H, Sehr T, Thomas K, Paul F, Richter N, et al. Safety and in vivo immune assessment of escalating doses of oral laquinimod in patients with RRMS. Journal of Neuroinflammation 2017;14(1):172. Additional file 1 [Figure S1, Figure S2, Figure S3, Table S1 Distribution of study drug termination reasons, Table S2, Table S3). - PMC - PubMed
References to studies excluded from this review
Barkhof 2018 {published data only}
-
- Barkhof F, Kappos L, Bar-Or A, Li D, Belachew S, Julian L, et al. Rapid onset of ocrelizumab suppression of brain MRI activity in relapsing-remitting multiple sclerosis. Multiple Sclerosis 2018;24:NP14-5.
Beutler 1996 {published data only}
Boiko 2018 {published data only}
-
- Boiko AN, Bosenko LP, Vasilovskii VV, Volkova LI, Zakharova MN, Kotov SV, et al. Comparative placebo-controlled clinical trial of the efficacy and safety of Interferon β-1a formulations for S.C. administration in patients with remitting multiple sclerosis: first-year results. Neuroscience and Behavioral Physiology 2018;48(7):883-9.
-
- Boyko AN, Bosenko LP, Vasilovskiy VV, Volkova LI, Zakharova MN, Kotov SV, et al. Efficacy, tolerability and safety of the treatment with teberif: the results of a 2-year randomized clinical trial of treatment naïve patients with remitting multiple sclerosis, who have not received DMT, after switching from other Interferon β-1a. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova 2019;119(2):73-85. - PubMed
Boyko 2019 {published data only}
-
- Boyko AN, Bosenko LP, Vasilovskiy VV, Volkova LI, Zakharova MN, Kotov SV, et al. Efficacy, tolerability and safety of the treatment with teberif: the results of a 2-year randomized clinical trial of treatment naïve patients with remitting multiple sclerosis, who have not received DMT, after switching from other interferon β-1a [Éffektivnost', perenosimost' i bezopasnost' terapii preparatom teberif: rezul'taty dvukhletnego klinicheskogo issledovaniia u patsientov s remittiruiushchim rasseiannym sklerozom, ranee ne poluchavshikh PITRS, i pri perekliuchenii s drugogo interferona β-1a]. Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova 2019;119(2. Vyp. 2):73-85. - PubMed
Boyko 2022 {published data only}
-
- Boyko AN, Bakhtiyarova KZ, Dudin VA, Zaslavsky LG, Malkova NA, Parshina YV, et al. The new pegylated Interferon beta-1a (sampegInterferon beta-1a, BCD-054) in the treatment of remitting multiple sclerosis. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova 2019;119(10):100-9. - PubMed
-
- Boyko AN, Boyko OV, Bakhtiyarova KZ, Gusev EI, Dudin VA, Zaslavsky LG, et al. Efficacy and safety of sampeginterferon β-1a in the treatment of relapsing remitting multiple sclerosis: results of 52 weeks of therapy in a randomized, double-blind clinical trial [Effektivnost' i bezopasnost' sampeginterferona β-1a dlya lecheniya remittiruyushchego rasseyannogo skleroza: rezul'taty 52-nedel'nogo randomizirovannogo dvoinogo slepogo klinicheskogo issledovaniya]. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 2022;122(1):62-71. - PubMed
Cohen 2009 {published data only}
-
- Cohen JA, Imrey PB, Calabresi PA, Edwards KR, Eickenhorst T, Felton WL 3rd, et al. Results of the Avonex Combination Trial (ACT) in relapsing-remitting MS. Neurology 2009;72(6):535-41. - PubMed
Cohen 2019 {published data only}
-
- Cohen JA, Campbell N, Wiendl H, Foley J, Butzkueven H, Ryerson LZ, et al. Evaluating the efficacy and safety of 6-week extended interval dosing of Natalizumab via a prospective, controlled, randomized phase 3B study. Multiple Sclerosis 2019;25:48-9.
-
- Foley J, Defer G, Zhovtis Ryerson L, Cohen JA, Arnold DL, Butzkueven H, et al. Baseline characteristics of multiple sclerosis patients enrolled in NOVA, a multicentre, randomised trial to assess the efficacy of Natalizumab every-6-weeks dosing. European Journal of Neurology 2020;27:212.
-
- NCT03689972. A study to evaluate efficacy, safety, and tolerability of EID of Natalizumab (BG00002) in participants with RRMS switching from treatment with Natalizumab SID in relation to continued SID treatment- followed by extension study comprising sc and iv Natalizumab administration [A randomized, controlled, open-label, rater-blinded, phase 3B study of the efficacy, safety, and tolerability of 6-week extended interval dosing (EID) of Natalizumab (BG00002) in subjects with relapsing-remitting multiple sclerosis switching from treatment with 4-week Natalizumab standard interval dosing (SID) in relation to continued SID treatment - followed by an open-label crossover extension study comprising subcutaneous and intravenous Natalizumab administration]. ClinicalTrials.gov/show/NCT03689972 (first received 1 October 2018).
Comi 2011 {published data only}
-
- Comi G, Cohen JA, Arnold DL, Wynn D, Filippi M, FORTE Study Group. Phase III dose-comparison study of glatiramer acetate for multiple sclerosis. Annals of Neurology 2011;69(1):75-82. - PubMed
Comi 2016 {published data only}
-
- Comi G, Freedman MS, Kappos L, Olsson TP, Miller AE, Wolinsky JS, et al. Pooled safety and tolerability data from four placebo-controlled teriflunomide studies and extensions. Multiple Sclerosis and Related Disorders 2016;5:97-104. - PubMed
CORAL 2006 {published data only}
-
- Filippi M, Wolinsky JS, Comi G, CORAL Study Group. Effects of oral glatiramer acetate on clinical and MRI-monitored disease activity in patients with relapsing multiple sclerosis: a multicentre, double-blind, randomised, placebo-controlled study. The Lancet Neurology 2006;5(3):213-20. - PubMed
DELIVER 2016 {published data only}
-
- NCT00559702. A randomized, open-label, dose-ranging study to evaluate the pharmacokinetics and initial safety of subcutaneous and intramuscular Natalizumab in subjects with multiple sclerosis. ClinicalTrials.gov/show/NCT00559702 (first received 16 November 2007).
-
- Plavina T, Fox EJ, Lucas N, Muralidharan KK, Mikol D. A randomized trial evaluating various administration routes of natalizumab in multiple sclerosis. Journal Of Clinical Pharmacology 2016;56(10):1254-62. - PubMed
Edan 1997 {published data only}
-
- Edan G, Miller D, Clanet M, Confavreux C, Lyon-Caen O, Lubetzki C, et al. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. Journal of Neurology, Neurosurgery and Psychiatry 1997;62(2):112-8. - PMC - PubMed
EPOC 2014 {published data only}
-
- Fox E, Edwards K, Burch G, Wynn DR, LaGanke C, Crayton H, et al. Outcomes of switching directly to oral fingolimod from injectable therapies: Results of the randomized, open-label, multicenter, Evaluate Patient OutComes (EPOC) study in relapsing multiple sclerosis. Multiple Sclerosis And Related Disorders 2014;3(5):607-19. - PubMed
-
- NCT01216072. A 6-month, randomized, open-label, patient outcomes, safety and tolerability study of fingolimod (FTY720) 0.5 mg/day vs. comparator in patients with relapsing forms of multiple sclerosis (EPOC) [A 6-month, randomized, active comparator, open-label, multi-center study to evaluate patient outcomes, safety and tolerability of Fingolimod (FTY720) 0.5 mg/day in patients with relapsing forms of multiple sclerosis who are candidates for MS therapy change from previous disease modifying therapy (EPOC)]. ClinicalTrials.gov/show/NCT01216072 (first received 7 October 2010).
EVOLVE‐MS‐1 2022 {published data only}
-
- NCT026 34307. A study of ALKS 8700 in adults with relapsing remitting multiple sclerosis (MS) EVOLVE-MS-1. ClinicalTrials.gov: NCT02634307 (first received 18 December 2015).
Freedman 2012 {published data only}
-
- Freedman MS, Wolinsky JS, Wamil B, Confavreux C, Comi G, Kappos L, et al. Teriflunomide added to interferon- in relapsing multiple sclerosis: a randomized phase II trial. Neurology 2012;78(23):1877-85. - PubMed
Freedman 2015 {published data only}
Gobbi 2013 {published data only}
-
- NCT01144052. Natalizumab de-escalation with Interferon beta-1b [De-escalation after Natalizumab treatment with Interferon-beta-1b in patients with relapsing-remitting multiple sclerosis]. ClinicalTrials.gov/show/NCT01144052 (first received 15 June 2010).
Goodman 2009 {published data only}
Hartung 2020 {published data only}
Hauser 2018 {published data only}
-
- Hauser SL, Kappos L, Montalban X, Koendgen H, Li C, Marcillat C, et al. Safety of Ocrelizumab in Multiple sclerosis: Updated Analysis in Patients With Relapsing and Primary Progressive Multiple sclerosis. European Journal of Neurology 2018;25:334.
Havrdova 2009 {published data only}
-
- Havrdova E, Zivadinov R, Krasensky J, Dwyer MG, Novakova I, Dolezal O, et al. Randomized study of interferon beta-1a, low-dose azathioprine, and low-dose corticosteroids in multiple sclerosis. Multiple Sclerosis 2009;15(8):965-76. - PubMed
Hu 2016 {published data only}
Hughes 2018 {published data only}
-
- Hughes R, Dalakas MC, Merkies I, Latov N, Léger JM, Nobile-Orazio E, et al. Oral fingolimod for chronic inflammatory demyelinating polyradiculoneuropathy (FORCIDP Trial): a double-blind, multicentre, randomised controlled trial. Lancet Neurology 2018;17(8):689-98. - PubMed
Kappos 2014 {published data only}
-
- Kappos L, Hartung HP, Freedman MS, Boyko A, Radü EW, Mikol DD, et al. Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurology 2014;13(4):353-63. - PubMed
-
- NCT00642902. A phase 2 study of Atacicept in subjects with relapsing multiple sclerosis (ATAMS) [A four-arm randomized, double-blind, placebo-controlled, multicenter phase ii study to evaluate the safety, tolerability and efficacy as assessed by frequent MRI measures of 3 doses of Atacicept monotherapy in subjects with relapsing multiple sclerosis (RMS) over a 36 week treatment course]. ClinicalTrials.gov/show/NCT00642902 (first received 25 March 2008).
Kappos 2016 {published data only}
-
- Kappos L, Li DK, Stüve O, Hartung HP, Freedman MS, Hemmer B, et al. Safety and efficacy of siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis: dose-blinded, randomized extension of the phase 2 BOLD study. JAMA Neurology 2016;73(9):1089-98. - PubMed
-
- NCT01185821. Long-term safety, tolerability and efficacy of BAF312 given orally in patients with relapsing-remitting multiple sclerosis [A dose blinded extension study to the CBAF312A2201 study to evaluate long-term safety, tolerability and efficacy of BAF312 given orally once daily in patients with relapsing-remitting multiple sclerosis]. ClinicalTrials.gov/show/NCT01185821 (first received 20 August 2010).
Kastrukoff 1990 {published data only}
-
- Kastrukoff LF, Oger JJ, Hashimoto SA, Sacks SL, Li DK, Palmer MR, et al. Systemic lymphoblastoid interferon therapy in chronic progressive multiple sclerosis. I. Clinical and MRI evaluation. Neurology 1990;40(3 Pt 1):479-86. - PubMed
Khoury 2010 {published data only}
Komori 2016 {published data only}
-
- NCT01212094. Double blind combination of rituximab by intravenous and intrathecal injection versus placebo in patients with low-inflammatory secondary progressive multiple sclerosis (RIVITaLISe). ClinicalTrials.gov/show/NCT01212094 (first received 30 September 2010).
Le Page 2015 {published data only}
-
- Le Page E, Veillard D, Laplaud DA, Hamonic S, Wardi R, Lebrun C, et al. Oral versus intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): A randomised, controlled, double-blind, non-inferiority trial. Lancet 2015;386:974-81. - PubMed
-
- NCT00984984. Efficacy and safety of Methylprednisolone per os versus iv for the treatment of multiple sclerosis (MS) relapses (COPOUSEP) [Randomised double-blinded trial comparing efficacy and safety of Methylprednisolone per os versus iv for the treatment of multiple sclerosis relapses]. ClinicalTrials.gov/show/NCT00984984 (first received 25 September 2009).
Mancardi 2015 {published data only}
-
- Mancardi GL, Sormani MP, Gualandi F, Saiz A, Carreras E, Merelli E, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a phase II trial. Neurology 2015;84(10):981-8. - PubMed
Mayer 2019 {published data only}
-
- Mayer L, Kappos L, Racke MK, Rammohan K, Traboulsee A, Hauser SL, et al. Ocrelizumab infusion experience in patients with relapsing and primary progressive multiple sclerosis: Results from the phase 3 randomized OPERA I, OPERA II, and ORATORIO studies. Multiple Sclerosis and Related Disorders 2019;30:236-43. - PubMed
Montalban 2019 {published data only}
-
- Montalban X, Arnold DL, Weber MS, Staikov I, Piasecka-Stryczynska K, Willmer J, et al. Evobrutinib phase 2 study group. Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis. The New England Journal of Medicine 2019;380(25):2406-17. - PubMed
-
- NCT02975349. A study of efficacy and safety of M2951 in participants with relapsing multiple sclerosis [A randomized, double-blind, placebo-controlled phase ii study of M2951 with a parallel, open-label, active control group (Tecfidera), in patients with relapsing multiple sclerosis to evaluate efficacy, safety, tolerability, pharmacokinetics, and biological activity]. ClinicalTrials.gov/show/NCT02975349 (first received 29 November 2016).
NCT00206648 {published data only}
-
- NCT00206648. An efficacy and safety comparison study of two marketed drugs in patients with relapsing-remitting MS (ABOVE) [A randomized, rater-blinded, multicenter, parallel-group study comparing the efficacy and safety of Betaseron 250 µg subcutaneously every other day with Avonex 30 µg intramuscularly once per week in relapsing-remitting multiple sclerosis patients previously treated with Avonex]. ClinicalTrials.gov/show/NCT00206648 (first received 21 September 2005).
NCT01058005 {published data only}
-
- NCT01058005. Study evaluating Rebif, Copaxone, and Tysabri for active multiple sclerosis (SURPASS) [A multicenter, randomized, open-label, parallel-group, active-controlled study to evaluate the benefits of switching therapy (glatiramer acetate or interferon beta-1a) to natalizumab in subjects with relapsing remitting multiple sclerosis (SURPASS)]. ClinicalTrials.gov/show/NCT01058005 (first received 28 January 2010).
NCT01065727 {published data only}
-
- NCT01065727. Impact study of 2 therapeutic strategy for aggressive remitting multiple sclerosis (IQUALYSEP) [Study impact, on clinical outcomes, quality of life and costs of 2 therapeutic strategy (monthly Natalizumab versus Mitoxantrone then immunomodulator) at 3 years of follow-up for aggressive remitting multiple sclerosis]. ClinicalTrials.gov/show/NCT01065727 (first received 9 February 2010).
NCT01337427 {published data only}
-
- NCT01337427. Using optical coherence tomography (OCT) to evaluate the efficacy and safety of Pegylated Interferon beta-1a (BIIB017) in patients with relapsing multiple sclerosis [Optical Coherence Tomography (OCT) in a multicenter, randomized,double-blind, parallel-group, placebo-controlled study to evaluate the efficacy and safety of Pegylated interferon beta-1a (BIIB017) in subjects with relapsing multiple sclerosis]. ClinicalTrials.gov/show/NCT01337427 (first received 18 April 2011).
Okai 2019 {published data only}
Perumal 2019 {published data only}
PREFERMS 2018 {published data only}
-
- Cascione M, Tenenbaum N, Wendt J, Meng X, Schofield L, Cree BACT. Treatment retention on Fingolimod compared with injectable multiple sclerosis therapies in African-American patients: a subgroup analysis of a randomized phase 4 study. Multiple Sclerosis and Related Disorders 2018;25:50-6. - PubMed
-
- Cree B, Cohen J, Silva D, Ritter S, Piani MD, Tomic D, et al. Confirmed disability improvement in patients treated with FINGOLIMOD in phase 3 and extension trial programmes for up to 96 months. Multiple Sclerosis 2017;23(3):322.
-
- NCT01623596. Evaluation of patient retention of Fingolimod vs. currently approved disease modifying therapy in patients with relapsing remitting multiple sclerosis. (PREFERMS) [A 12-month, prospective, randomized, active-controlled, open-label study to evaluate the patient retention of Fingolimod vs. approved first-line disease modifying therapies in adults with relapsing remitting multiple sclerosis (PREFERMS)]. ClinicalTrials.gov/show/NCT01623596 (first arrived 20 June 2012).
Rahimdel 2015 {published data only}
Ramo‐Tello 2014 {published data only}
-
- NCT00753792. Oral corticotherapy in megadoses to treat multiple sclerosis during relapse [Multicenter, randomized, double blind, clinical trial to compare the clinical and radiological efficacy of equivalent doses of Methylprednisolone administered orally or intravenously in patients with multiple sclerosis during relapse]. ClinicalTrials.gov/show/NCT00753792 (first received 17 September 2008).
-
- Ramo-Tello C, Grau-López L, Tintoré M, Rovira A, Ramió i Torrenta L, Brieva L, et al. A randomized clinical trial of oral versus intravenous methylprednisolone for relapse of MS. Multiple Sclerosis (Houndmills, Basingstoke, England) 2014;20(6):717-25. - PubMed
-
- Ramo-Tello C, Tintoré M, Rovira A, Ramió-Torrenta L, Brieva L, Saiz A, et al. Baseline clinical status as a predictor of methylprednisolone response in multiple sclerosis relapses. Multiple Sclerosis 2016;22:117-21. - PubMed
RESTORE 2014 {published data only}
-
- NCT01071083. Treatment interruption of Natalizumab (RESTORE) [Randomized treatment interruption of Natalizumab]. ClinicalTrials.gov/show/NCT01071083 (first received 19 February 2010).
Rice 2000 {published data only}
-
- Rice GP, Filippi M, Comi G. Cladribine and progressive MS: clinical and MRI outcomes of a multicenter controlled trial. Cladribine MRI Study Group. Neurology 2000;54(5):1145-55. - PubMed
Rieckmann 2012 {published data only}
-
- NCT01142466. A phase IV study of Rebif ® 44mcg administered three times per week by subcutaneous injection compared with no treatment in the therapy of relapsing multiple sclerosis after mitoxantrone (REMAIN) [Phase IV, multicenter, open label, randomized study of Rebif® 44mcg administered three times per week by subcutaneous injection compared with no treatment in the therapy of relapsing multiple sclerosis after Mitoxantrone]. ClinicalTrials.gov/show/NCT01142466 (first received 11 June 2010).
-
- Rieckmann P, Heidenreich F, Sailer M, Zettl UK, Zessack N, Hartung HP, et al. Treatment de-escalation after mitoxantrone therapy: results of a phase IV, multicentre, open-label, randomized study of subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis. Therapeutic Advances In Neurological Disorders 2012;5(1):3-12. - PMC - PubMed
RIVITALISE 2016 {published data only}
-
- NCT01212094. Double blind combination of rituximab by intravenous and intrathecal injection versus placebo in patients with low-inflammatory secondary progressive multiple sclerosis (RIVITaLISe). clinicaltrials.gov/show/NCT01212094 (firstreceived 30 September 2010).
Romine 1999 {published data only}
-
- Romine JS, Sipe JC, Koziol JA, Zyroff J, Beutler E. A double-blind, placebo-controlled, randomized trial of cladribine in relapsing-remitting multiple sclerosis. Proceedings of the Association of American Physicians 1999;111(1):35-44. - PubMed
Saida 2016 {published data only}
-
- Saida T, Kira JI, Ueno Y, Harada T. Long-term efficacy and safety of intramuscular interferon beta-1a: Randomized postmarketing trial of two dosing regimens in Japanese patients with relapsing-remitting multiple sclerosis. Multiple Sclerosis and Related Disorders 2016;7:102-8. - PubMed
SELECTION 2014 {published data only}
-
- Giovannoni G, Gold R, Selmaj K, Havrdova E, Montalban X, Radue EW, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECTION): a multicentre, randomised, double-blind extension trial. The Lancet. Neurology 2014;13(5):472-81. - PubMed
-
- NCT00870740. Safety and efficacy extension study of daclizumab high yield process (DAC HYP) in participants with multiple sclerosis who have completed study 205MS201 (NCT00390221) to treat relapsing-remitting multiple sclerosis (SELECTION) [A double-blind, multicenter, extension study to evaluate the safety and efficacy of DAC HYP in subjects with multiple sclerosis who have completed treatment in study 205MS201 (SELECT)]. ClinicalTrials.gov/show/NCT00870740 (first received 27 March 2009).
SENTINEL 2006 {published data only}
-
- NCT00030966. Safety and efficacy of Natalizumab in combination with Avonex in the treatment of multiple sclerosis [A randomized, double-blind, placebo-controlled, parallel-group, multicenter study to determine the safety and efficacy of Natalizumab, when added to Avonex® (Interferon beta-1a), in subjects with relapsing-remitting multiple sclerosis]. ClinicalTrials.gov/show/NCT00030966 (first received 18 February 2002).
-
- Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. New England Journal of Medicine 2006;354(9):911-23. - PubMed
Sipe 1994 {published data only}
-
- Sipe JC, Romine JS, Koziol JA, McMillan R, Zyroff J, Beutler E. Cladribine in treatment of chronic progressive multiple sclerosis. Lancet 1994;344(8914):9-13. - PubMed
Sorensen 2014 {published data only}
-
- NCT00640328. Ofatumumab dose-finding in relapsing remitting multiple sclerosis (RRMS) patients (OMS115102) [A double-blind, randomized, placebo controlled, multicenter, dose-finding trial of Ofatumumab in relapsing remitting multiple sclerosis (RRMS) patients]. clinicaltrials.gov/show/NCT00640328 (first received 21 March 2008).
-
- Sorensen PS, Lisby S, Grove R, Derosier F, Shackelford S, Havrdova E, et al. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology 2014;82(7):573-81. - PubMed
Sorensen 2017 {published data only}
Stelmasiak 2000 {published data only}
-
- Stelmasiak Z, Bartosik-Psujek H, Belniak-Legiec E, Mitosek-Szewczyk K. The effect of cladribine on some parameters of blood and cerebrospinal fluid in patients with relapsing-remitting multiple sclerosis (RR-MS). Annales Universitatis Mariae Curie-Sklodowska 2000;55:221-5. - PubMed
Tahara 2020 {published data only}
-
- Tahara M, Oeda T, Okada K, Kiriyama T, Ochi K, Maruyama H, et al. Safety and efficacy of Rituximab in neuromyelitis optica spectrum disorders (RIN-1 study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurology 2020;19(4):298-306. - PubMed
Trojano 2015 {published data only}
-
- Trojano M, Ramio-Torrenta L, Grimaldi L, Lubetzki C, Schippling S, Evans K, et al. A randomized, blinded, parallel-group phase-2 study exploring the efficacy, safety, and tolerability of multiple natalizumab dosing regimens in patients with relapsing multiple sclerosis (REFINE). European Journal of Neurology 2015;22:49.
Turner 2019 {published data only}
Wolinsky 2015 {published data only}
-
- NCT01874145. Safety and tolerability of Glatiramer acetate (GLACIER) [An open-label, randomized, multi-center, parallel-arm study to assess the safety and tolerability of Glatiramer acetate 40 mg/ml three times a week compared to 20 mg/ml daily subcutaneous injections in subjects with relapsing-remitting multiple sclerosis]. ClinicalTrials.gov/show/NCT01874145 (first received 10 June 2013).
-
- Wolinsky JS, Borresen TE, Dietrich DW, Wynn D, Sidi Y, Steinerman JR, et al. GLACIER: An open-label, randomized, multicenter study to assess the safety and tolerability of glatiramer acetate 40 mg three-times weekly versus 20 mg daily in patients with relapsing-remitting multiple sclerosis. Multiple Sclerosis and Related Disorders 2015;4:370-6. - PubMed
-
- Wynn D, Kolodny S, Rubinchick S, Steinerman J, Knappertz V, Wolinsky J. Patient experience with glatiramer acetate 40 mg/1 ml three-times weekly treatment for relapsing-remitting multiple sclerosis: Results from the GLACIER extension study. Neurology 2015;84:S14.
Wray 2019 {published data only}
Wynn 2010 {published data only}
-
- NCT00109161. Study of subcutaneous Daclizumab in patients with active, relapsing forms of multiple sclerosis [A phase II randomized, double-blinded, placebo-controlled, multi-center study of subcutaneous Daclizumab in patients with active, relapsing forms of multiple sclerosis]. ClinicalTrials.gov/show/NCT00109161 (first received 25 April 2005).
-
- Wynn D, Kaufman M, Montalban X, Vollmer T, Simon J, Elkins J, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurology 2010;9(4):381-90. - PubMed
References to ongoing studies
EudraCT 2018‐000284‐93 {published data only}
-
- A multinational, multicenter, randomized, Phase III, double blind, parallel group, placebo controlled study in subjects with relapsing forms of MS to assess the efficacy, safety and tolerability of GA Depot, a long acting IM injection of glatiramer acetate, administered once every four weeks. https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-000284-93/EE/.
NCT04035005 {published data only}
-
- A study to evaluate the efficacy and safety of Ocrelizumab in adults with primary progressive multiple sclerosis (O'HAND) [A phase IIIB multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of Ocrelizumab in adults with primary progressive multiple sclerosis]. ClinicalTrials.gov/show/NCT04035005 (first received 29July 2019).
NCT04121403 {published data only}
-
- Norwegian study of oral Cladribine and Rituximab in Multiple Sclerosis (NOR-MS) [Norwegian study of oral Cladribine and Rituximab in multiple sclerosis (NOR-MS) a prospective randomized open-label blinded endpoint (PROBE) multicenter non-inferiority study]. ClinicalTrials.gov/show/NCT04121403 (first received 9 October 2019).
NCT04578639 {published data only}
-
- Ocrelizumab VErsus Rituximab Off-Label at the Onset of Relapsing MS Disease (OVERLORD-MS) [Ocrelizumab versus Rituximab off-label at the onset of relapsing MS]. ClinicalTrials.gov/show/NCT04578639 (first received 8 October 2020).
NCT04688788 {published data only}
-
- Non-inferiority study of Ocrelizumab and Rituximab in active Multiple Sclerosis (DanNORMS) [Danish non-inferiority study of Ocrelizumab and Rituximab in MS (DanNORMS): a randomized study comparing the efficacy of Ocrelizumab and Rituximab in active multiple sclerosis]. ClinicalTrials.gov/show/NCT04688788 (first received 30 December 2020).
NCT04695080 {published data only}
-
- ChariotMS - Cladribine to halt deterioration in people with advanced multiple sclerosis (ChariotMS) [ChariotMS - A national (UK) phase IIB, multi-centre, randomised, double-blind, placebo controlled (1:1) efficacy trial with cost-utility analysis of Cladribine tablets (3.5mg/kg over two years) in people with advanced multiple sclerosis. Is Cladribine superior to placebo in protecting upper limb function?]. ClinicalTrials.gov/show/NCT04695080 (first received 5 January 2021).
NCT04788615 {published data only}
-
- NCT04788615. Open label randomized multicenter to assess efficacy & tolerability of Ofatumumab 20mg vs. first line DMT in RMS (STHENOS) [Open-label rater-blind randomized multi-center parallel-arm active- comparator study to assess the efficacy and tolerability of Ofatumumab 20mg sc monthly vs. first line DMT - physician's choice in the treatment of newly diagnosed RMS]. ClinicalTrials.gov/show/NCT04788615 (first received 9 March 2021).
NCT05090371 {published data only}
-
- NCT05090371. A multicenter study of continued current therapy vs transition to Ofatumumab after neurofilament (NFL) elevation (SOSTOS) [A randomized, open label, multi-center, active-comparator study to assess efficacy, safety & tolerability of Ofatumumab 20mg sc monthly versus continued current therapy in relapsing-remitting multiple sclerosis after elevation of serum neurofilament light levels (SOSTOS)]. ClinicalTrials.gov/show/NCT05090371 (first received 22 October 2021).
RAMBLE 2021 {published data only}
-
- ACTRN12621001502820. Reducing the frequency of autoimmune adverse events in the treatment of multiple sclerosis with alemtuzumab using B-cell depletion (RAMBLE): a phase II, randomised, placebo-controlled clinical trial. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12621001502820 2021.
Additional references
Amato 2015
-
- Amato MP, Portaccio E. Fertility, pregnancy and childbirth in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs 2015;29(3):207-20. - PubMed
Bender 2008
-
- Bender R, Bunce C, Clarke M, Gates S, Lange S, Pace NL, Thorlund K. Attention should be given to multiplicity issues in systematic reviews. Journal of Clinical Epidemiology 2008;61:857-65. - PubMed
Bomprezzi 2015
Bourdette 2016
-
- Bourdette D. Rituximab for treating multiple sclerosis: off-label but on target. Neurology 2016;87:2070-1. - PubMed
Brignardello‐Petersen 2021
-
- Brignardello-Petersen R, Guyatt GH, Mustafa RA, Chu DK, Hultcrantz M, Schünemann HJ, Tomlinson G. GRADE guidelines 33: Addressing imprecision in a network meta-analysis. J Clin Epidemiol 2021;139:49-56. - PubMed
Chaimani 2012
-
- Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161-76. - PubMed
Chaimani 2013
Chen 2005
-
- Chen T, Hoppe FM. Simultaneous confidence intervals. In: In: Armitage P, Colton T (editors). Encyclopedia of Biostatistics (2nd edition). Chichester (UK): John Wiley & Sons, 2005, 2005.
Chouhfeh 2015
-
- Chouhfeh L, Kavak KS, Teter BE, Weinstock-Guttman B. Disease modifying therapies use associated with comorbid autoimmune diseases in multiple sclerosis patients. Multiple Sclerosis and Related Disorders 2015;4(3):228-33. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. - PubMed
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Statistics in Medicine 08 March 2010;Volume 29(Issue 7-8):932-44. - PubMed
Farber 2015
FDA 2020
-
- CFR - Code of Federal Regulations Title 21. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=312.32 (accessed 27 October 2020).
Federal Register 2015
-
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. https://www.federalregister.gov/articles/2014/12/04/2014-28241/content-a... (accessed 18 April 2016).
Filippini 2013
-
- Filippini G, Del Giovane C, Vacchi L, D'Amico R, Di Pietrantonj C, Beecher D, Salanti G. Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD008933. [DOI: 10.1002/14651858.CD008933.pub2] - DOI - PMC - PubMed
Garattini 2013
-
- Garattini S, Bertelé V, Banzi R. Placebo? no thanks, it might be bad for me! European Journal of Clininical Pharmacology 2013;69(3):711-4. - PubMed
Glanville 2019
-
- Glanville Julie, Foxlee Ruth, Wisniewski Susi, Noel-Storr Anna, Edwards Mary, Dooley Gordon. Translating the Cochrane EMBASE RCT filter from the Ovid interface to Embase.com: a case study. Health Information & Libraries Journal 2019;36(3):264-277. - PubMed
Glanville 2019a
-
- Glanville Julie, Dooley Gordon, Wisniewski Susi, Foxlee Ruth, Noel-Storr Anna. Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information & Libraries Journal 2019;36(1):73-90. - PubMed
Goodman 2006
-
- Goodman LS, Gilman A, Brunton LL, Lazo JS, Parker KL. Goodman & Gilman's the pharmacological basis of therapeutics. New York: McGraw-Hill, 2006.
GRADE Working Group 2004
Hamidi 2018
Hartung 2021
-
- Hartung HP, Meuth SG, Thompson AJ. Paradigm shifts: Early initiation of high-efficacy disease-modifying treatment in multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2021;27(10):1473-6. - PubMed
Higgins 2011
-
- Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane-handbook.org.
ICH 2015
-
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Introductory guide MedDRA version 18.0. http://www.meddra.org/sites/default/files/guidance/file/intguide_18_0_en... (accessed 18 April 2016).
Ioannidis 2004
-
- Ioannidis JP, Evans SJ, Gøtzsche PC, O'Neill RT, Altman DG, Schulz K, et al, CONSORT Group. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781-8. - PubMed
Jackson 2014
Kanters 2016
Lebrun 2018
-
- Lebrun C, Rocher F. Cancer risk in patients with multiple sclerosis: potential impact of disease-modifying drugs. CNS Drugs 2018;32(10):939-49. - PubMed
Lefebvre 2022
-
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). The Cochrane Collaboration, 2022. Available from training.cochrane.org/handbook.
Li 2020
-
- Li H, Hu F, Zhang Y, Li K. Comparative efficacy and acceptability of disease-modifying therapies in patients with relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. Journal of Neurology 2020;267(12):3489-98. - PubMed
Lopez‐Leon 2020
Lublin 1996
-
- Lublin F, Reingold S. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46:907-11. - PubMed
Lublin 2014
Lucchetta 2019
-
- Lucchetta RC, Leonart LP, Becker J, Pontarolo R, Fernandez-Llimós F, Wiens A. Safety outcomes of disease-modifying therapies for relapsing-remitting multiple sclerosis: A network meta-analysis. Multiple Sclerosis and Related Disorders 2019;35:7-15. - PubMed
Lycke 2015
Manríquez 2008
-
- Manríquez Juan J. A highly sensitive search strategy for clinical trials in Literatura Latino Americana e do Caribe em Ciências da Saúde (LILACS) was developed. Journal of Clinical Epidemiology 2008;61(4):407-11. - PubMed
Marshall 2018
Martìnez‐Càceres 1998
-
- Martínez-Cáceres EM, Río J, Barrau M, Durán I, Borrás C, Tintoré M, et al. Amelioration of flulike symptoms at the onset of interferon beta-1b therapy in multiple sclerosis by low-dose oral steroids is related to a decrease in interleukin-6 induction. Annals of Neurology 1998;44(4):682-5. - PubMed
McCool 2019
-
- McCool R, Wilson K, Arber M, Fleetwood K, Toupin S, Thom H, et al. Systematic review and network meta-analysis comparing ocrelizumab with other treatments for relapsing multiple sclerosis. Multiple Sclerosis and Related Disorders 2019;29:55-61. - PubMed
McDonald 2001
-
- McDonald W, Compston A, Edan G, Goodkin D, Hartung H, Lublin F. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50:121-7. - PubMed
McDonald 2017
-
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit; 2017 September 13-16; Cape Town, South Africa. 2017.
Miladinovic 2014
-
- Miladinovic B, Hozo I, Chaimani A, Djulbegovic B. Indirect treatment comparison. Stata Journal 2014;14(1):76–86.
Montalban 2018
-
- Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Multiple Sclerosis 2018;24(2):96-120. - PubMed
Multiple‐Treatments Meta‐analysis (MTM)
-
- Multiple-Treatments Meta-analysis (MTM). A framework for evaluating and ranking multiple healthcare technologies. http://www.mtm.uoi.gr/ (accessed 18 April 2016).
Nikolakopoulou 2018
Nikolakopoulou 2020
Noel‐Storr 2018
-
- Noel-Storr AH and the Project Transform Team. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 June 18-20; Oxford, UK. 2018.
Peryer 2020
-
- Peryer G, Golder S, Junqueira D, Vohra S, Loke YK. Chapter 19: Adverse effects. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020.. Available from www.training.cochrane.org/handbook.
Peters 2008
-
- Peters J, Sutton A, Jones D, Abrams K, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991-6. - PubMed
Polman 2005
-
- Polman C, Reingold S, Edan G, Filippi M, Hartung H, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the 'McDonald Criteria'. Annals of Neurology 2005;58:840-6. - PubMed
Polman 2011
Poser 1983
-
- Poser C, Paty D, Scheinberg L, McDonald W, Davis F, Ebers G, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13:227-31. - PubMed
Prosperini 2020
Rae‐Grant 2018
-
- Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis. Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018;90(17):777-88. - PubMed
Rucker 2012
-
- Rücker G. Network meta-analysis, electrical networks and graph theory. Research Synthesis Methods 2012;3:312-24. - PubMed
Rucker 2015
Salanti 2011
-
- Salanti G, Ades A, Ioannidis J. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64:163-71. - PubMed
Salanti 2012
-
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80–97. - PubMed
Samjoo 2020
-
- Samjoo IA, Worthington E, Drudge C, Zhao M, Cameron C, Häring DA, et al. Comparison of ofatumumab and other disease-modifying therapies for relapsing multiple sclerosis: a network meta-analysis. Journal of Comparative Effectiveness Research 2020;9(18):1255-74. - PubMed
Schwrtzer 2015
-
- Schwarzer G, Carpenter GR, Rücker G. Meta-Analysis with R. In: Meta-Analysis with R. Springer, 2015.
Schünemann 2011
-
- Schünemann H, Oxman A, Higgins J, Vist G, Glasziou P, Guyatt G. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane-handbook.org.
Siddiqui 2018
-
- Siddiqui MK, Khurana IS, Budhia S, Hettle R, Harty G, Wong SL. Systematic literature review and network meta-analysis of cladribine tablets versus alternative disease-modifying treatments for relapsing-remitting multiple sclerosis. Current Medical Research and Opinion 2018;34(8):1361-71. - PubMed
Silva 2022
-
- Silva GD, Castrillo BB, Apóstolos-Pereira SL, Callegaro D. Is there a role for off-label high-efficacy disease-modifying drugs in progressive multiple sclerosis? A network meta-analysis. Acta Neurol Scandinavica 2022;146(5):403-9. - PubMed
Simpson 2021
Thomas 2017
-
- Thomas J, Noel-Storr A, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living systematic reviews: 2. Combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. - PubMed
Tramacere 2015
-
- Tramacere I, Del Giovane C, Salanti G, D'Amico R, Filippini G. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011381. [DOI: 10.1002/14651858.CD011381.pub2] - DOI - PMC - PubMed
Van Assche 2005
-
- Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. New England Journal of Medicine 2005;353(4):362-8. - PubMed
Walton 2020
White 2011
-
- White IR. Multivariate random-effects meta-regression: updates to mvmeta. The STATA Journal 2011;11:255-70.
White 2012
Williamson 2015
-
- Williamson EM, Berger JR. Central nervous system infections with immunomodulatory therapies. Continuum (Minneapolis Minn.) 2015;21(6 Neuroinfectious Disease):1577-98. - PubMed
Xu 2018
-
- Xu X, Chi S, Wang Q, Li C, Xu B, Zhang J, et al. Efficacy and safety of monoclonal antibody therapies for relapsing remitting multiple sclerosis: A network meta-analysis. Multiple Sclerosis and Related Disorders 2018;25:322-8. - PubMed
Yepes‐Nuñez 2019
-
- Yepes-Nuñez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta-analysis. Journal of Clinical Epidemiology 2019;115:1-13. - PubMed
Zhang 2019
Zhang 2021
Zorzela 2016
-
- Zorzela L, Loke YK, Ioannidis JP, Golder S, Santaguida P, Altman DG, et al. PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ 2016;352:i157. - PubMed
Śladowska 2022
-
- Śladowska K, Kawalec P, Holko P, Osiecka O. Comparative safety of high-efficacy disease-modifying therapies in relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. Neurological sciences 2022;43(9):5479-500. - PubMed
References to other published versions of this review
Tramacere 2016
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

