Post-transcriptional capping generates coenzyme A-linked RNA
- PMID: 38032240
- PMCID: PMC10761072
- DOI: 10.1080/15476286.2023.2288740
Post-transcriptional capping generates coenzyme A-linked RNA
Abstract
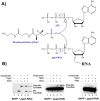
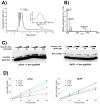
NAD can be inserted co-transcriptionally via non-canonical initiation to form NAD-RNA. However, that mechanism is unlikely for CoA-linked RNAs due to low intracellular concentration of the required initiator nucleotide, 3'-dephospho-CoA (dpCoA). We report here that phosphopantetheine adenylyltransferase (PPAT), an enzyme of CoA biosynthetic pathway, accepts RNA transcripts as its acceptor substrate and transfers 4'-phosphopantetheine to yield CoA-RNA post-transcriptionally. Synthetic natural (RNAI) and small artificial RNAs were used to identify the features of RNA that are needed for it to serve as PPAT substrate. RNAs with 4-10 unpaired nucleotides at the 5' terminus served as PPAT substrates, but RNAs having <4 unpaired nucleotides did not undergo capping. No capping was observed when the +1A was changed to G or when 5' triphosphate was removed by RNA pyrophosphohydrolase (RppH), suggesting the enzyme recognizes pppA-RNA as an ATP analog. PPAT binding affinities were equivalent for transcripts with +1A, +1 G, or 5'OH (+1A), indicating that productive enzymatic recognition is driven more by local positioning effects than by overall binding affinity. Capping rates were independent of the number of unpaired nucleotides in the range of 4-10 nucleotides. Capping was strongly inhibited by ATP, reducing CoA-RNA production ~70% when equimolar ATP and substrate RNA were present. Dual bacterial expression of candidate RNAs with different 5' structures followed by CoA-RNA CaptureSeq revealed 12-fold enrichment of the better PPAT substrate, consistent with in vivo CoA-capping of RNA transcripts by PPAT. These results suggest post-transcriptional RNA capping as a possible mechanism for the biogenesis of CoA-RNAs in bacteria.
Keywords: CoA-RNA; CoA-RNA CaptureSeq; PPAT; cofactor-RNA conjugates; post-transcriptional CoA-capping.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
