Formation and function of multiciliated cells
- PMID: 38032388
- PMCID: PMC10689204
- DOI: 10.1083/jcb.202307150
Formation and function of multiciliated cells
Abstract
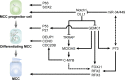
In vertebrates, multiciliated cells (MCCs) are terminally differentiated cells that line the airway tracts, brain ventricles, and reproductive ducts. Each MCC contains dozens to hundreds of motile cilia that beat in a synchronized manner to drive fluid flow across epithelia, the dysfunction of which is associated with a group of human diseases referred to as motile ciliopathies, such as primary cilia dyskinesia. Given the dynamic and complex process of multiciliogenesis, the biological events essential for forming multiple motile cilia are comparatively unelucidated. Thanks to advancements in genetic tools, omics technologies, and structural biology, significant progress has been achieved in the past decade in understanding the molecular mechanism underlying the regulation of multiple motile cilia formation. In this review, we discuss recent studies with ex vivo culture MCC and animal models, summarize current knowledge of multiciliogenesis, and particularly highlight recent advances and their implications.
© 2023 Lyu et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

