Electrifying Friedel-Crafts Intramolecular Alkylation toward 1,1-Disubstituted Tetrahydronaphthalenes
- PMID: 38032548
- PMCID: PMC10729024
- DOI: 10.1021/acs.joc.3c01281
Electrifying Friedel-Crafts Intramolecular Alkylation toward 1,1-Disubstituted Tetrahydronaphthalenes
Abstract
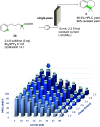
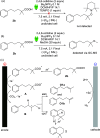
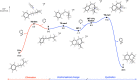
In this work, we successfully employed electrochemical conditions to promote a Hofer-Moest, intramolecular Friedel-Crafts alkylation sequence. The reaction proceeds under mild conditions, employing carboxylic acids as starting materials. Notably, the electrochemical process performed in batch was adapted to a continuous flow electrolysis apparatus to provide a significant improvement. This catalyst-free, electrochemical approach produces an array of tetrahydronaphthalenes that could be used for API synthesis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Friedel C.; Friedel J. M. The alkylation or acylation of aromatic compounds catalysed by aluminium chloride or other Lewis acids. Compt. Rend. 1877, 84, 1392–1395.
- Friedel C.; Crafts J. M. A New General Synthetical Method of Producing Hydrocarbons. J. Chem. Soc. 1877, 32, 725–791.
-
To prepare amyl iodide, Friedel and Crafts treated amyl chloride with aluminum and iodide using benzene as the solvent. Instead of amyl iodide, they ended up with amylbenzene. Unlike others before them who may have simply discarded the reaction, they thoroughly investigated the Lewis acid-catalyzed alkylations and acylations, and a powerful reaction was born.
-
- Olah G. A.; Reddy V. P.; Prakash G. K. S.. Friedel-Crafts Reactions. In Encyclopedia of Chemical Technology; Wiley, 2000.
- Macquarrie D. J.Industrial Friedel–Crafts Chemistry. In Catalytic Asymmetric Friedel-Crafts Alkylations; Bandini M., Umani- Ronchi A., Eds.; Wiley Online Books, 2009; pp 271–288.
- Surburg H.; Panten J.. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 6th ed.; Wiley-VCH Verlag, 2016; Vol. 105.
- Franck H. G.Industrial Aromatic Chemistry; Springer: Berlin, 1988.
- Price C. C. The Alkylation of Aromatic Compounds by the Friedel-Crafts Method. Org. React. 1946, 3, 1.10.1002/0471264180.or003.01. - DOI
- Roberts R. M.; Khalaf A. A.. Friedel-Crafts Alkylation Chemistry: A Century of Discovery; Marcel Dekker Inc.: New York, 1984.
- Eyley S. C. The Aliphatic Friedel– Crafts Reaction. Compr. Org. Synth. 1991, 2, 707–731. 10.1016/B978-0-08-052349-1.00045-7. - DOI
-
- Constable D. J. C.; Dunn P. J.; Hayler J. D.; Humphrey G. R.; Leazer J. L. Jr.; Linderman R. J.; Lorenz K.; Manley J.; Pearlman B. A.; Wells A.; Zaks A.; Zhang T. Y. Key green chemistry research areas—a perspective from pharmaceutical manufacturers. Green Chem. 2007, 9, 411–420. 10.1039/B703488C. - DOI
-
- Rueping M.; Nachtsheim B. J. A review of new developments in the Friedel–Crafts alkylation – From green chemistry to asymmetric catalysis. Beilstein J. Org. Chem. 2010, 6, 6.and references cited therein10.3762/bjoc.6.6. - DOI - PMC - PubMed
- Poulsen T. B.; Jørgensen K. A. Catalytic Asymmetric Friedel–Crafts Alkylation Reactions—Copper Showed the Way. Chem. Rev. 2008, 108, 2903–2915. 10.1021/cr078372e. - DOI - PubMed
- Kumar V.; Turnbull W. B.; Kumar A. Review on Recent Developments in Biocatalysts for Friedel–Crafts Reactions. ACS Catal. 2022, 12, 10742–10763. and references cited therein10.1021/acscatal.2c01134. - DOI
- Leveson-Gower R. B.; Roelfes G. Biocatalytic Friedel-Crafts Reactions. ChemCatChem 2022, 14, e20220063610.1002/cctc.202200636. - DOI - PMC - PubMed
- Sartori G.; Maggi R. Use of Solid Catalysts in Friedel–Crafts Acylation Reactions. Chem. Rev. 2006, 106, 1077–1104. and references cited therein10.1021/cr040695c. - DOI - PubMed
-
- Yan M.; Kawamata Y.; Baran P. S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319. 10.1021/acs.chemrev.7b00397. - DOI - PMC - PubMed
- Wiebe A.; Gieshoff T.; Möhle S.; Rodrigo E.; Zirbes M.; Waldvogel S. R. Electrifying Organic Synthesis. Angew. Chem., Int. Ed. 2018, 57, 5594–5619. 10.1002/anie.201711060. - DOI - PMC - PubMed
- Medici F.; Resta S.; Andolina S.; Benaglia M. Recent Advances in Enantioselective Catalytic Electrochemical Organic Transformations. Catalysts 2023, 13, 944.10.3390/catal13060944. - DOI
- Meyer T. H.; Choi I.; Tian C.; Ackermann L. Powering the Future: How Can Electrochemistry Make a Difference in Organic Synthesis?. Chem. 2020, 6, 2484–2496. 10.1016/j.chempr.2020.08.025. - DOI
- Kingston C.; Palkowitz M. D.; Takahira Y.; Vantourout J. C.; Peters B. K.; Kawamata Y.; Baran P. S. A Survival Guide for the “Electro-curious. Acc. Chem. Res. 2020, 53, 72–83. 10.1021/acs.accounts.9b00539. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

