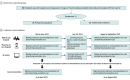
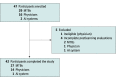
Conflict of Interest Disclosures: Dr Sunami reported receiving personal fees from Chugai Pharmaceutical Co Ltd, Illumina Inc, Guardant Health Japan, and MDS KK and grant funding from Sysmex Corporation outside the submitted work. Dr Naito reported receiving grant funding from the Ministry of Health, Labour and Welfare and personal fees from the Genomedia Inc–National Cancer Center joint research fund during the conduct of the study; grant funding from AbbVie Inc, Ono Pharmaceutical Co Ltd, Daiichi Sankyo, Pfizer Inc, Boehringer Ingelheim, Eli Lilly and Company, Eisai Co Ltd, AstraZeneca, Chugai Pharmaceutical Co Ltd, Bayer AG, and Genomedia Inc outside the submitted work; and lecture fees from AstraZeneca, Eisai Co Ltd, Ono Pharmaceutical Co Ltd, Guardant Health Japan, Takeda Pharmaceuticals International, Eli Lilly and Company, Novartis AG, Pfizer Inc, Chugai Pharmaceutical Co Ltd, FUJIFILM Toyama Chemical Co Ltd, Taiho Pharmaceutical Co Ltd, Mundipharma International Limited, Bristol Myers Squibb, and Shionogi & Company, Ltd outside the submitted work. Dr Ennishi reported receiving personal fees from Nippon Shinyaku Co Ltd and honoraria for lectures and personal fees from Chugai Pharmaceutical Co Ltd, Eisai Co Ltd, Bristol Myers Squibb, and Kyowa Kirin Co Ltd outside the submitted work. Dr Imai reported receiving support from Sumitomo Corporation for participation in academic conferences outside the submitted work and having had a US patent provisional application pending. Dr Kage reported receiving grant funding from Konica Minolta Inc outside the submitted work. Dr Kanai reported receiving personal fees from Chugai Pharmaceutical Co Ltd, having stock ownership in Therabiopharma, receiving research expenses from Molecular Health GmbH outside the submitted work, and having US patents 11141529B2, 6923162, and r6931853 issued. Dr Kenmotsu reported receiving grant funding from Chugai Pharmaceutical Co Ltd and personal fees from Chugai Pharmaceutical Co Ltd during the conduct of the study and receiving grant funding from AstraZeneca, Eli Lilly and Company, Daiichi Sankyo, Ono Pharmaceutical Co Ltd, and Novartis AG and personal fees from AstraZeneca, Taiho Pharmaceutical Co Ltd, Eli Lilly and Company, Boehringer Ingelheim, Bristol Myers Squibb Japan, Kyowa Kirin Co Ltd, MSD KK, Daiichi Sankyo, Pfizer Inc, Merck & Co Inc, Nippon Kayaku, Bayer Yakuhin Ltd, Amgen Inc, Takeda Pharmaceuticals International, Ono Pharmaceutical Co Ltd, and Loxo Oncology Inc outside the submitted work. Dr Komine reported receiving personal fees from Chugai Pharmaceutical Co Ltd, Incyte Corporation, Kyowa Kirin Co Ltd, Merck & Co Inc, Taiho Pharmaceutical Co Ltd, Takeda Pharmaceuticals International, and Daiichi Sankyo, outside the submitted work. Dr Koyama reported receiving grant funding from PACT Pharma Inc, Chugai Pharmaceutical Co Ltd, Daiichi Sankyo, Novartis AG, Eli Lilly and Company, Pfizer Inc, Janssen Pharmaceuticals, Zymeworks Inc, Takeda Pharmaceuticals International, AstraZeneca, and Noile-Immune Biotech Inc and personal fees from Chugai Pharmaceutical Co Ltd, AstraZeneca, and Sysmex Corporation outside the submitted work. Dr Sakai reported receiving grants from Eli Lilly and Company, Chugai Pharmaceutical Co Ltd, and Daiichi Sankyo outside the submitted work. Dr Kozuki reported receiving personal fees from Chugai Pharmaceutical Co Ltd, AstraZeneca, Ono Pharmaceutical Co Ltd, Eli Lilly and Company Japan, Bristol Myers Squibb, MSD KK, Taiho Pharmaceutical Co Ltd, Nippon Boehringer Ingelheim, Nippon Kayaku, Daiichi Sankyo, Merck & Co Inc, Pfizer Inc Japan, Takeda Pharmaceuticals International, Amgen Inc, Novartis AG, Bayer AG, AbbVie Inc, Kyowa Kirin Co Ltd, and Sawai Pharmaceutical Co Ltd, and grant funding from Chugai Pharmaceutical Co Ltd, AstraZeneca, Eli Lilly and Company Japan, Taiho Pharmaceutical Co Ltd, Bristol Myers Squibb, Ono Pharmaceutical Co Ltd, MSD KK, Kyowa Kirin Co Ltd, Merck & Co Inc, Daiichi Sankyo, Amgen Inc, AbbVie Inc, Sanofi SA, Eisai Co Ltd, Labcorp Japan, IQVIA Services Japan KK, Gilead Sciences Inc, Pfizer Inc, and Bayer AG outside the submitted work. Dr Sakashita reported receiving grant funding from Ono Pharmaceutical Co Ltd, Chugai Pharmaceutical Co Ltd, AstraZeneca KK, Taiho Pharmaceutical Co Ltd, Boehringer Ingelheim Japan Inc, Novartis Pharma KK, Merck & Co Inc, and SBI Pharmaceuticals Co Ltd, and personal fees from Ono Pharmaceutical Co Ltd, Chugai Pharmaceutical Co Ltd, AstraZeneca KK, Taiho Pharmaceutical Co Ltd, Boehringer Ingelheim Japan Inc, Daiichi Sankyo, Nippon Kayaku, Novartis Pharma KK, Astellas Pharma Inc, and Merck & Co Inc outside the submitted work. Dr Takashima reported receiving grant funding from Daiichi Sankyo, Pfizer Inc, Ono Pharmaceutical Co Ltd, MSD KK, Bristol Myers Squibb, Isofol Medical, Hutchison Medipharma Ltd, and Incyte Corporation and personal fees from Eli Lilly and Company, Ono Pharmaceutical Co Ltd, Taiho Pharmaceutical Co Ltd, Chugai Pharmaceutical Co Ltd, Takeda Pharmaceuticals International, and Merck Serono outside the submitted work. Dr Kubo reported receiving personal fees from Bristol Myers Squibb, Taiho Pharmaceutical Co Ltd, Kyowa Kirin Co Ltd, AstraZeneca, Ono Pharmaceutical Co Ltd, Nippon Kayaku, Chugai Pharmaceutical Co Ltd, MSD KK, Pfizer Inc, Eli Lilly and Company, Novartis AG, Boehringer Ingelheim, and Towa Pharmaceutical Co Ltd outside the submitted work. Dr Segawa reported receiving honoraria for lectures from Taiho Pharmaceutical Co Ltd during the conduct of the study. Dr Bando reported receiving lecture fees from Eli Lilly and Company Japan, Taiho Pharmaceutical Co Ltd, and Ono Pharmaceutical Co Ltd and grant funding from Ono Pharmaceutical Co Ltd outside the submitted work. Dr Makiyama reported receiving personal fees from Eli Lilly and Company Japan, Taiho Pharmaceutical Co Ltd, Ono Pharmaceutical Co Ltd, Bristol Myers Squibb, and Daiichi Sankyo outside the submitted work. Dr Kinoshita reported receiving personal fees from Chugai Pharmaceutical Co Ltd, Takeda Pharmaceuticals International, MSD KK, Bayer AG, and AstraZeneca KK and nonfinancial support from Novartis Pharma KK outside the submitted work. Dr Ohe reported receiving grant funding from AstraZeneca, Chugai Pharmaceutical Co Ltd, Eli Lilly and Company, Ono Pharmaceutical Co Ltd, BMS, Takeda Pharmaceuticals International, Janssen Pharmaceuticals, and AnHeart Therapeutics Inc and personal fees from AstraZeneca, Celltrion Inc, Chugai Pharmaceutical Co Ltd, Ono Pharmaceutical Co Ltd, BMS, Pfizer Inc, Taiho Pharmaceutical Co Ltd, Takeda Pharmaceuticals International, Janssen Pharmaceuticals, AnHeart Therapeutics Inc, Novartis AG, and Amgen Inc outside the submitted work. Dr Ishioka reported receiving personal fees from Kyowa Kirin Co Ltd, Daiichi Sankyo, Eli Lilly and Company, Nippon Kayaku, Takeda Pharmaceuticals International, Taiho Pharmaceutical Co Ltd, Chugai Pharmaceutical Co Ltd, Incyte Corporation, AstraZeneca, Merck & Co Inc, BMS, Bayer AG, M3 Inc, MSD KK, Novartis AG, and Ono Pharmaceutical Co Ltd and grant funding from Hitachi Ltd, Daiichi Sankyo, Kyowa Kirin Co Ltd, Nippon Kayaku, Asahi Kasei, Taiho Pharmaceutical Co Ltd, and Chugai Pharmaceutical Co Ltd outside the submitted work. Dr Yamamoto reported receiving personal fees from Chugai Pharmaceutical Co Ltd, CMIC Holdings Co Ltd, J-Pharma Co Ltd, Craif Inc, Johokiko Co Ltd, Triceps, Kanagawa Prefectural Hospital Organization, and Kanagawa Medical Practitioners Association and grant funding from Taiho Pharmaceutical Co Ltd, Boehringer Ingelheim, Ono Pharmaceutical Co Ltd, Takeda Pharmaceuticals International, Bayer Yakuhin Ltd, Daiichi Sankyo, Astellas Pharma Inc, and Kyowa Kirin Co Ltd outside the submitted work. Dr Tsuchihara reported receiving personal fees from Takeda Pharmaceuticals International, Chugai Pharmaceutical Co Ltd, Eisai Co Ltd, Boehringer Ingelheim, Bayer AG, Miyarisan Pharmaceutical Co Ltd, and Illumina Inc outside the submitted work. Dr Yoshino reported receiving grant funding from the Ministry of Health, Labour and Welfare during the conduct of the study and receiving grant funding from Amgen KK, Chugai Pharmaceutical Co Ltd, Daiichi Sankyo, Eisai Co Ltd, FALCO Biosystems Ltd, Genomedia Inc, Molecular Health GmbH, Nippon Boehringer Ingelheim, Ono Pharmaceutical Co Ltd, Pfizer Inc Japan, Roche Diagnostics KK, Sanofi KK, Sysmex Corporation, Taiho Pharmaceutical Co Ltd, Parexel International, and MSD KK; honoraria from Bayer Yakuhin Ltd, Merck Biopharma, Chugai Pharmaceutical Co Ltd, MSD KK, Ono Pharmaceutical Co Ltd, and Takeda Pharmaceuticals International; and consulting fees from Sumitomo Corporation outside the submitted work. No other disclosures were reported.