Sustained hyperglycemia specifically targets translation of mRNAs for insulin secretion
- PMID: 38032734
- PMCID: PMC10849759
- DOI: 10.1172/JCI173280
Sustained hyperglycemia specifically targets translation of mRNAs for insulin secretion
Abstract
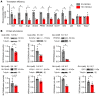
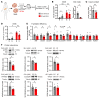
Pancreatic β cells are specialized for coupling glucose metabolism to insulin peptide production and secretion. Acute glucose exposure robustly and coordinately increases translation of proinsulin and proteins required for secretion of mature insulin peptide. By contrast, chronically elevated glucose levels that occur during diabetes impair β cell insulin secretion and have been shown experimentally to suppress insulin translation. Whether translation of other genes critical for insulin secretion is similarly downregulated by chronic high glucose is unknown. Here, we used high-throughput ribosome profiling and nascent proteomics in MIN6 insulinoma cells to elucidate the genome-wide impact of sustained high glucose on β cell mRNA translation. Before induction of ER stress or suppression of global translation, sustained high glucose suppressed glucose-stimulated insulin secretion and downregulated translation of not only insulin, but also mRNAs related to insulin secretory granule formation, exocytosis, and metabolism-coupled insulin secretion. Translation of these mRNAs was also downregulated in primary rat and human islets following ex vivo incubation with sustained high glucose and in an in vivo model of chronic mild hyperglycemia. Furthermore, translational downregulation decreased cellular abundance of these proteins. Our study uncovered a translational regulatory circuit during β cell glucose toxicity that impairs expression of proteins with critical roles in β cell function.
Keywords: Beta cells; Endocrinology; Islet cells; Metabolism; Translation.
Conflict of interest statement
Figures






Update of
-
Sustained hyperglycemia specifically targets translation of mRNAs for insulin secretion.bioRxiv [Preprint]. 2023 Sep 29:2023.09.29.560203. doi: 10.1101/2023.09.29.560203. bioRxiv. 2023. Update in: J Clin Invest. 2023 Nov 30;134(3):e173280. doi: 10.1172/JCI173280. PMID: 37808767 Free PMC article. Updated. Preprint.
References
-
- [No authors listed] Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the Diabetes Control and Complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann Intern Med. 1998;128(7):517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

