SARS-CoV-2 Epidemiology and COVID-19 mRNA Vaccine Effectiveness Among Infants and Children Aged 6 Months-4 Years - New Vaccine Surveillance Network, United States, July 2022-September 2023
- PMID: 38032834
- PMCID: PMC10718202
- DOI: 10.15585/mmwr.mm7248a2
SARS-CoV-2 Epidemiology and COVID-19 mRNA Vaccine Effectiveness Among Infants and Children Aged 6 Months-4 Years - New Vaccine Surveillance Network, United States, July 2022-September 2023
Abstract
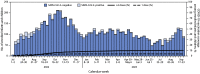
SARS-CoV-2 infection in young children is often mild or asymptomatic; however, some children are at risk for severe disease. Data describing the protective effectiveness of COVID-19 mRNA vaccines against COVID-19-associated emergency department (ED) visits and hospitalization in this population are limited. Data from the New Vaccine Surveillance Network, a prospective population-based surveillance system, were used to estimate vaccine effectiveness using a test-negative, case-control design and describe the epidemiology of SARS-CoV-2 in infants and children aged 6 months-4 years during July 1, 2022-September 30, 2023. Among 7,434 children included, 5% received a positive SARS-CoV-2 test result, and 95% received a negative test result; 86% were unvaccinated, 4% had received 1 dose of any vaccine product, and 10% had received ≥2 doses. When compared with receipt of no vaccines among children, receipt of ≥2 COVID-19 mRNA vaccine doses was 40% effective (95% CI = 8%-60%) in preventing ED visits and hospitalization. These findings support existing recommendations for COVID-19 vaccination of young children to reduce COVID-19-associated ED visits and hospitalization.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Janet A. Englund reports institutional support from AstraZeneca, GSK, Pfizer-BioNTech, and Merck & Co. and consulting fees from Abbvie, AstraZeneca, Ark Biopharma, Meissa Vaccines, Moderna, Sanofi Pasteur, Shinogi, GSK, and Pfizer-BioNTech. John V. Williams reports institutional support from the National Institutes of Health (NIH) and compensation for service on Quidel’s Scientific Advisory Board through 2022 and on GSK’s Independent Data Monitoring Committee. Marian G. Michaels reports support from the National Institute of Allergy and Infectious Diseases, NIH, and Merck & Co.; receipt of lecture honoraria from the Transplant Alliance; support for meeting attendance from NIH, the Infectious Diseases Society of America, the Transplant Alliance, the American Society of Transplantation, and the International Transplant Nurses Association; participation on a National Institute of Allergy and Infectious Diseases Data Safety Monitoring Board; and services as Chair of the Infectious Diseases Committee of the International Pediatric Transplant Association. Geoffrey A. Weinberg reports institutional support from the New York State Department of Health AIDS Institute and honoraria from Merck & Co. for writing and editing textbook chapters in the Merck & Co. Manual. Natasha B. Halasa reports grants from Sanofi, Quidel, and Merck & Co. Elizabeth P. Schlaudecker reports institutional support from NIH and Pfizer-BioNTech, support for attending a Pediatric Infectious Diseases Society meeting, uncompensated service on NIH Data Safety Monitoring Board, honorarium for service on Sanofi Pasteur advisory board, uncompensated membership in the World Society of Pediatric Infectious Diseases, and uncompensated service as committee chair for the Pediatric Infectious Diseases Society. Mary Allen Staat reports institutional support from NIH, Merck & Co., Cepheid, and Pfizer-BioNTech and receipt of royalties from UpToDate. Peter G. Szilagyi reports a grant to the University of Rochester (subcontract to University of California at Los Angeles) No other potential conflicts of interest were disclosed.
Figures
References
-
- Fleming-Dutra KE, Wallace M, Moulia DL, et al. Interim recommendations of the Advisory Committee on Immunization Practices for use of Moderna and Pfizer-BioNTech COVID-19 vaccines in children aged 6 months–5 years—United States, June 2022. MMWR Morb Mortal Wkly Rep 2022;71:859–68. 10.15585/mmwr.mm7126e2 - DOI - PubMed
-
- Fleming-Dutra KE, Ciesla AA, Roper LE, et al. Preliminary estimates of effectiveness of monovalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection among children aged 3–5 years—Increasing Community Access to Testing Program, United States, July 2022–February 2023. MMWR Morb Mortal Wkly Rep 2023;72:177–82. 10.15585/mmwr.mm7207a3 - DOI - PMC - PubMed
-
- Link-Gelles R, Ciesla AA, Rowley EAK, et al. Effectiveness of monovalent and bivalent mRNA vaccines in preventing COVID-19–associated emergency department and urgent care encounters among children aged 6 months–5 years—VISION Network, United States, July 2022–June 2023. MMWR Morb Mortal Wkly Rep 2023;72:886–92. 10.15585/mmwr.mm7233a2 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous