Deciphering and disrupting PIEZO1-TMEM16F interplay in hereditary xerocytosis
- PMID: 38033286
- PMCID: PMC10862370
- DOI: 10.1182/blood.2023021465
Deciphering and disrupting PIEZO1-TMEM16F interplay in hereditary xerocytosis
Abstract
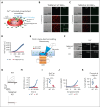
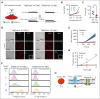
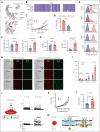
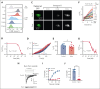
Cell-surface exposure of phosphatidylserine (PS) is essential for phagocytic clearance and blood clotting. Although a calcium-activated phospholipid scramblase (CaPLSase) has long been proposed to mediate PS exposure in red blood cells (RBCs), its identity, activation mechanism, and role in RBC biology and disease remain elusive. Here, we demonstrate that TMEM16F, the long-sought-after RBC CaPLSase, is activated by calcium influx through the mechanosensitive channel PIEZO1 in RBCs. PIEZO1-TMEM16F functional coupling is enhanced in RBCs from individuals with hereditary xerocytosis (HX), an RBC disorder caused by PIEZO1 gain-of-function channelopathy. Enhanced PIEZO1-TMEM16F coupling leads to an increased propensity to expose PS, which may serve as a key risk factor for HX clinical manifestations including anemia, splenomegaly, and postsplenectomy thrombosis. Spider toxin GsMTx-4 and antigout medication benzbromarone inhibit PIEZO1, preventing force-induced echinocytosis, hemolysis, and PS exposure in HX RBCs. Our study thus reveals an activation mechanism of TMEM16F CaPLSase and its pathophysiological function in HX, providing insights into potential treatment.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for Y.Z. is Shenzhen Bay Laboratory, Guangdong, China.
Figures


Comment in
-
Mechanosensation and lipid scrambling news.Blood. 2024 Jan 25;143(4):300-301. doi: 10.1182/blood.2023023367. Blood. 2024. PMID: 38270947 No abstract available.
References
-
- Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39(1):407–427. - PubMed
-
- Bevers EM, Williamson PL. Getting to the outer leaflet: physiology of phosphatidylserine exposure at the plasma membrane. Physiol Rev. 2016;96(2):605–645. - PubMed
-
- Kay JG, Grinstein S. Phosphatidylserine-mediated cellular signaling. Adv Exp Med Biol. 2013;991:177–193. - PubMed
-
- Krahling S, Callahan MK, Williamson P, Schlegel RA. Exposure of phosphatidylserine is a general feature in the phagocytosis of apoptotic lymphocytes by macrophages. Cell Death Differ. 1999;6(2):183–189. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

