Recent advancements in iodide/phosphine-mediated photoredox radical reactions
- PMID: 38033449
- PMCID: PMC10682514
- DOI: 10.3762/bjoc.19.131
Recent advancements in iodide/phosphine-mediated photoredox radical reactions
Abstract
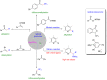
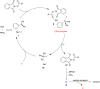
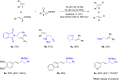
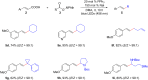
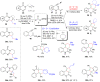
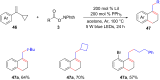
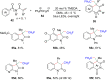
Photoredox catalysis plays a crucial role in contemporary synthetic organic chemistry. Since the groundbreaking work of Shang and Fu on photocatalytic decarboxylative alkylations in 2019, a wide range of organic transformations, such as alkylation, alkenylation, cyclization, amination, iodination, and monofluoromethylation, have been progressively achieved using a combination of iodide and PPh3. In this review, we primarily focus on summarizing the recent advancements in inexpensive and readily available iodide/phosphine-mediated photoredox radical transformations.
Keywords: annulation; decarboxylative; iodide/phosphine; photocatalytic; radical reaction.
Copyright © 2023, Liu et al.
Figures




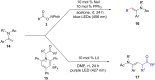
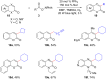

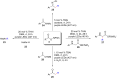


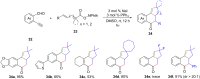







References
Publication types
LinkOut - more resources
Full Text Sources
