Lipids and lipoproteins may play a role in the neuropathology of Alzheimer's disease
- PMID: 38033552
- PMCID: PMC10687420
- DOI: 10.3389/fnins.2023.1275932
Lipids and lipoproteins may play a role in the neuropathology of Alzheimer's disease
Abstract
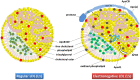
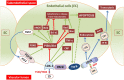
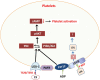
Alzheimer's disease (AD) and other classes of dementia are important public health problems with overwhelming social, physical, and financial effects for patients, society, and their families and caregivers. The pathophysiology of AD is poorly understood despite the extensive number of clinical and experimental studies. The brain's lipid-rich composition is linked to disturbances in lipid homeostasis, often associated with glucose and lipid abnormalities in various neurodegenerative diseases, including AD. Moreover, elevated low-density lipoprotein (LDL) cholesterol levels may be related to a higher probability of AD. Here, we hypothesize that lipids, and electronegative LDL (L5) in particular, may be involved in the pathophysiology of AD. Although changes in cholesterol, triglyceride, LDL, and glucose levels are seen in AD, the cause remains unknown. We believe that L5-the most electronegative subfraction of LDL-may be a crucial factor in understanding the involvement of lipids in AD pathology. LDL and L5 are internalized by cells through different receptors and mechanisms that trigger separate intracellular pathways. One of the receptors involved in L5 internalization, LOX-1, triggers apoptotic pathways. Aging is associated with dysregulation of lipid homeostasis, and it is believed that alterations in lipid metabolism contribute to the pathogenesis of AD. Proposed mechanisms of lipid dysregulation in AD include mitochondrial dysfunction, blood-brain barrier disease, neuronal signaling, inflammation, and oxidative stress, all of which lead ultimately to memory loss through deficiency of synaptic integration. Several lipid species and their receptors have essential functions in AD pathogenesis and may be potential biomarkers.
Keywords: Alzheimer’s disease; LDLR; LOX-1; cholesterol; electronegative LDL; lipids.
Copyright © 2023 Akyol, Akyol, Chou, Chen, Liu, Selek, Soares and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abe Y., Fornage M., Yang C. Y., Bui-Thanh N. A., Wise V., Chen H. H., et al. . (2007). L5, the most electronegative subfraction of plasma LDL, induces endothelial vascular cell adhesion molecule 1 and CXC chemokines, which mediate mononuclear leukocyte adhesion. Atherosclerosis 192, 56–66. doi: 10.1016/j.atherosclerosis.2006.06.012 - DOI - PubMed
-
- Akyol O., Chowdhury I., Akyol H. R., Tessier K., Vural H., Akyol S. (2020). Why are cardiovascular diseases more common among patients with severe mental illness? The potential involvement of electronegative low-density lipoprotein (LDL) L5. Med. Hypotheses 142:109821. doi: 10.1016/j.mehy.2020.109821 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

