The relationship between miR-21, DNA methylation, and bisphenol a in bovine COCs and granulosa cells
- PMID: 38033863
- PMCID: PMC10684922
- DOI: 10.3389/fcell.2023.1294541
The relationship between miR-21, DNA methylation, and bisphenol a in bovine COCs and granulosa cells
Abstract
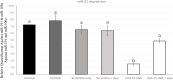
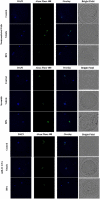
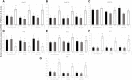
Introduction: miR-21 is a critical microRNA for the regulation of various processes in oocytes and granulosa cells. It is involved in the modulation of apoptosis and can influence other epigenetic mechanisms. Among these mechanisms, DNA methylation holds significant importance, particularly during female gametogenesis. Evidence has demonstrated that microRNAs, including miR-21, can regulate DNA methylation. Bisphenol A (BPA) is a widespread chemical that disrupts oocyte maturation and granulosa cell function. Recent findings suggested that BPA can act through epigenetic pathways, including DNA methylation and microRNAs. Methods: This study uses anti-miR-21 LNAs to explore the involvement of miR-21 in the regulation of DNA methylation in bovine Cumulus-Oocyte-Complexes (COCs) and granulosa cells, in the presence and absence of BPA. This study investigated 5 mC/5hmC levels as well as gene expression of various methylation enzymes using qPCR and western blotting. Results and discussion: Results reveal that BPA reduces 5mC levels in granulosa cells but not in COCs, which can be attributed to a decrease in the methylating enzymes DNMT1 and DNMT3A, and an increase in the demethylating enzyme TET2. We observed a significant increase in the protein levels of DNMT1, DNMT3A, and TET2 upon inhibition of miR-21 in both COCs and granulosa cells. These findings directly imply a strong correlation between miR-21 signaling and the regulation of DNA methylation in bovine COCs and granulosa cells under BPA exposure.
Keywords: DNA methylation; bisphenol A; granulosa cells; miR-21; oocytes.
Copyright © 2023 Sabry, May and Favetta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









Similar articles
-
Evidence for TET-mediated DNA demethylation as an epigenetic alteration in cumulus granulosa cells of women with polycystic ovary syndrome.Mol Hum Reprod. 2022 Jun 30;28(7):gaac019. doi: 10.1093/molehr/gaac019. Mol Hum Reprod. 2022. PMID: 35640568
-
Are BPA effects on Connexins and key downstream microRNAs in bovine COCs correlated to miR-21 expression?Reprod Biol. 2025 Jun;25(2):101026. doi: 10.1016/j.repbio.2025.101026. Epub 2025 May 20. Reprod Biol. 2025. PMID: 40398114
-
MicroRNA-130b is involved in bovine granulosa and cumulus cells function, oocyte maturation and blastocyst formation.J Ovarian Res. 2017 Jun 19;10(1):37. doi: 10.1186/s13048-017-0336-1. J Ovarian Res. 2017. PMID: 28629378 Free PMC article.
-
Effects of bisphenol A and bisphenol S on microRNA expression during bovine (Bos taurus) oocyte maturation and early embryo development.Reprod Toxicol. 2021 Jan;99:96-108. doi: 10.1016/j.reprotox.2020.12.001. Epub 2020 Dec 4. Reprod Toxicol. 2021. PMID: 33285269
-
Epigenetic effects of environmental chemicals bisphenol A and phthalates.Int J Mol Sci. 2012;13(8):10143-10153. doi: 10.3390/ijms130810143. Epub 2012 Aug 15. Int J Mol Sci. 2012. PMID: 22949852 Free PMC article. Review.
Cited by
-
DNA methylation, but not microRNA expression, is affected by in vitro THC exposure in bovine granulosa cells.BMC Pharmacol Toxicol. 2024 Jul 15;25(1):42. doi: 10.1186/s40360-024-00763-5. BMC Pharmacol Toxicol. 2024. PMID: 39010179 Free PMC article.
References
-
- Bhandari R. K., Taylor J. A., Sommerfeld-Sager J., Tillitt D. E., Ricke W. A., Vom Saal F. S. (2019). Estrogen receptor 1 expression and methylation of Esr1 promoter in mouse fetal prostate mesenchymal cells induced by gestational exposure to bisphenol A or ethinylestradiol. Environ. Epigenet 5, dvz012–8. 10.1093/EEP/DVZ012 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

