Systematic Literature Review of Risk Factors for Poor Outcomes Among Adults With Respiratory Syncytial Virus Infection in High-Income Countries
- PMID: 38033988
- PMCID: PMC10686344
- DOI: 10.1093/ofid/ofad513
Systematic Literature Review of Risk Factors for Poor Outcomes Among Adults With Respiratory Syncytial Virus Infection in High-Income Countries
Abstract
Identification of risk factors for severe respiratory syncytial virus (RSV) disease in adults could facilitate their appropriate vaccine recommendations. We conducted a systematic literature review (last 10 years in PubMed/Embase) to identify quantitative estimates of risk factors for severe RSV infection outcomes in high-income countries. Severe outcomes from RSV infection included hospitalization, excess mortality, lower respiratory tract infection, or a composite measure: severe RSV, which included these outcomes and others, such as mechanical ventilation and extended hospital stay. Among 1494 articles screened, 26 met eligibility criteria. We found strong evidence that the following increased the risk of severe outcomes: age, preexisting comorbid conditions (eg, cardiac, pulmonary, and immunocompromising diseases, as well as diabetes and kidney disease), and living conditions (socioeconomic status and nursing home residence). The frequency of severe outcomes among younger adults with comorbidities was generally similar to that experienced by older adults, suggesting that immunosenescence and chronic conditions are both contributing factors for elevated risk.
Trial registration: PROSPERO (CRD42022315239).
Keywords: respiratory syncytial virus; risk factors; severe.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. D. C., S. K., B. D. G., and E. B. are employees of Pfizer & Co and may own Pfizer stock. Pfizer & Co is developing an adult vaccine for prevention of RSV infection. A. N., W. N., M. L., A. M., and J. M. are employees of RTI Health Solutions, which received funding from Pfizer & Co for this work, including manuscript development.
Figures
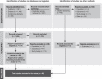


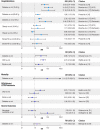
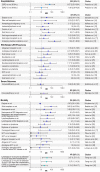
References
-
- Food and Drug Administration . FDA approves first respiratory syncytial virus (RSV) vaccine. 3 May 2023. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-r.... Accessed 19 July 2023.
-
- Pfizer . US FDA approves ABRYSVO, Pfizer's vaccine for the prevention of respiratory syncytial virus (RSV) in older adults. 31 May 2023. Available at: https://www.pfizer.com/news/press-release/press-release-detail/us-fda-ap.... Accessed 19 July 2023.
LinkOut - more resources
Full Text Sources
Miscellaneous

