Pseudohypoadrenalism, a subclinical cortisol metabolism disorder in hyperuricemia
- PMID: 38034015
- PMCID: PMC10687422
- DOI: 10.3389/fendo.2023.1279205
Pseudohypoadrenalism, a subclinical cortisol metabolism disorder in hyperuricemia
Abstract
Background: Hyperuricemia is a known risk factor of lipid metabolism disorder. However, the mechanisms have not been fully understood.
Methods: The serum samples from hyperuricemia subjects were used to analyze the correlation between serum uric acid and clinical characteristics. Hyperuricemia mice induced by potassium oxonate (PO) and adenine were used to explore glucocorticoid metabolism.
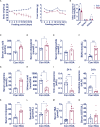
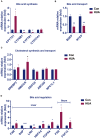
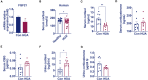
Results: In hyperuricemia patients, the levels of serum uric acid were positively correlated with the levels of γ-glutamyltransferase, associated with a cortisol metabolism disorder. In hyperuricemia state, the adrenal glands failed to respond to adrenocorticotropic hormone properly, leading to low cortisol, but not corticosterone production, and decreased mRNA levels of aldosterone synthase, 11β-hydroxylase, and 3β-hydroxysteroid dehydrogenase 1, three key enzymes for cortisol synthesis. The expression of both hepatic 5α-reductase and renal 11β-hydroxysteroid dehydrogenase 2 was significantly reduced, which led to low cortisol clearance. We denominated this cortisol metabolism disorder in hyperuricemia as pseudohypoadrenalism (PHAL).
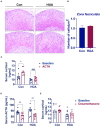
Conclusion: PHAL increased exposure to the bioavailable cortisol in the liver, leading to local amplification of the biological action of corticosteroids. Unregulated biosynthesis pathway of bile acid expanded bile acid pool, and further aggravated cholestatic liver injury.
Keywords: cortisol; hyperuricemia; lipid metabolism disorder; liver injury; pseudohypoadrenalism.
Copyright © 2023 Bao, Chen, Pan, Wang, Yu, Chen, Zhang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Growth hormone, insulin-like growth factor-I and the cortisol-cortisone shuttle.Horm Res. 2001;56 Suppl 1:1-6. doi: 10.1159/000048126. Horm Res. 2001. PMID: 11786677 Review.
-
Altered cortisol metabolism in polycystic ovary syndrome: insulin enhances 5alpha-reduction but not the elevated adrenal steroid production rates.J Clin Endocrinol Metab. 2003 Dec;88(12):5907-13. doi: 10.1210/jc.2003-030240. J Clin Endocrinol Metab. 2003. PMID: 14671189
-
Cortisol metabolism and the role of 11beta-hydroxysteroid dehydrogenase.Best Pract Res Clin Endocrinol Metab. 2001 Mar;15(1):61-78. doi: 10.1053/beem.2000.0119. Best Pract Res Clin Endocrinol Metab. 2001. PMID: 11469811 Review.
-
Molecular biology of 11β-hydroxylase and 11β-hydroxysteroid dehydrogenase enzymes.J Steroid Biochem Mol Biol. 1992 Dec;43(8):827-35. doi: 10.1016/0960-0760(92)90309-7. J Steroid Biochem Mol Biol. 1992. PMID: 22217826 Review.
-
Endogenous inhibitors of 11beta-hydroxysteroid dehydrogenase type 1 do not explain abnormal cortisol metabolism in polycystic ovary syndrome.Clin Endocrinol (Oxf). 2000 Jan;52(1):77-80. doi: 10.1046/j.1365-2265.2000.00893.x. Clin Endocrinol (Oxf). 2000. PMID: 10651756
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

