Efficient regulatory approval of two novel HIV prevention interventions in a resource-limited setting: experiences from Zimbabwe
- PMID: 38034413
- PMCID: PMC10682080
- DOI: 10.3389/frph.2023.1279124
Efficient regulatory approval of two novel HIV prevention interventions in a resource-limited setting: experiences from Zimbabwe
Abstract
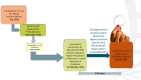
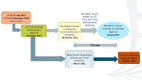
The global burden of HIV remains unacceptably high despite significant progress made in HIV treatment and prevention. There is an urgent need to scale up the comprehensive HIV prevention strategies that include pre-exposure prophylaxis (PrEP). Oral PrEP is highly effective in preventing HIV acquisition when taken regularly, but this remains a challenge for some at-risk individuals. Therefore, there is a need for other HIV prevention options. The dapivirine vaginal ring (DVR) and long-acting injectable cabotegravir (CAB-LA) are novel biomedical interventions that are safe and efficacious for HIV pre-exposure prophylaxis, as demonstrated in recently completed clinical trials. Timely roll-out and scalability of efficacious interventions depend on the registration process with the national medicine regulatory authorities (NMRAs). The Medicines Control Authority of Zimbabwe (MCAZ) was the first NMRA globally to approve the DVR in July 2021 and the first in Africa to approve CAB-LA for HIV prevention in July 2022. The regulatory review process for DVR and CAB-LA by MCAZ took 4.5 and 5.5 months, respectively. This efficient review process of the two interventions by MCAZ, a regulatory body in a resource-limited setting, provides important lessons to shorten timelines between the completion of the clinical development process and the registration of essential medicines.
Keywords: HIV; Zimbabwe; dapivirine vaginal ring; long-acting injectable cabotegravir; pre-exposure prophylaxis; regulatory approval.
© 2023 Murombedzi, Chirinda, Chareka, Chirenje and Mgodi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declared that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- HIV PREVENTION 2025—ROAD MAP: Getting on track to end AIDS as a public health threat by 2030. Geneva: Joint United Nations Programme on HIV/AIDS (2022). Licence: CC BY-NC-SA 3.0 IGO.
-
- The path that ends AIDS: UNAIDS Global AIDS Update 2023. Geneva. Joint United Nations Programme on HIV/AIDS (2023). Licence: CC BY-NC-SA 3.0 IGO.
-
- Ministry of Health and Child Care (MoHCC). Zimbabwe Population-based HIV Impact Assessment 2020 (ZIMPHIA 2020). Final Report. Harare: MoHCC (December 2021).
-
- World Health Organization. Pre-exposure prophylaxis (PrEP). WHO expands recommendation on oral PrEP of HIV infection (2015); (November): 2. Available from: http:apps.who.int/iris/bitstream/10665/197906/1/WHO_HIV_2015.48_eng.pdf?... (Accessed August 4, 2023).
-
- Mgodi NM, Murewanhema G, Moyo E, Samba C, Musuka G, Dzinamarira T, et al. Advancing the use of long-acting extended delivery formulations for HIV prevention in sub-Saharan Africa: challenges, opportunities, and recommendations. J Int AIDS Soc. (2023) 26:e26115. 10.1002/jia2.26115 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous